
Jon Renholds
National Network of Environmental Management Studies Fellow
December 1998
U.S. Environmental Protection Agency
Office of Solid Waste and Emergency Response
Technology Innovation Office
Washington, D.C.
This document was prepared by a National Network of Environmental Management Studies grantee under a fellowship from the U.S. Environmental Protection Agency. This report was not subject to EPA peer review or technical review. The U.S. EPA makes no warranties, expressed or implied, including without limitation, warranty for completeness, accuracy, or usefulness of the information, warranties as to the merchantability, or fitness for a particular purpose. Moreover, the listing of any technology, corporation, company, person, or facility in this report does not constitute endorsement, approval, or recommendation by the U.S. EPA.
Environmental concern and interest is growing for contaminated sediments, primarily due to the technological challenges for managing and remediating the sediment contamination. EPA’s Technology Innovation Office (TIO) provided a grant through the National Network for Environmental Management Studies (NNEMS) to prepare a technology assessment report on in situ treatment technologies to clean up contaminated sediments. This report was prepared by a senior undergraduate student from Oregon State University during the summer of 1998. It has been reproduced to help provide federal agencies, states, consulting engineering firms, private industries, and technology developers with information on the current status of this technology.
About the National Network for Environmental Management Studies (NNEMS)
NNEMS is a comprehensive fellowship program managed by the Environmental Education Division of EPA. The purpose of the NNEMS Program is to provide students with practical research opportunities and experiences.
Each participating headquarters or regional office develops and sponsors projects for student research. The projects are narrow in scope to allow the student to complete the research by working full-time during the summer or part-time during the school year. Research fellowships are available in Environmental Policy, Regulations, and Law; Environmental Management and Administration; Environmental Science; Public Relations and Communications; and Computer Programming and Development.
NNEMS fellows receive a stipend determined by the student’s level of education and the duration of the research project. Fellowships are offered to undergraduate and graduate students. Students must meet certain eligibility criteria.
This report is intended to provide a basic summary and current status of in situ treatment technologies for contaminated sediments. It contains information gathered from a range of currently available sources, including project documents, reports, periodicals, Internet searches, and personal communication with involved parties. No attempts were made to independently confirm the resources used.
While the original report included color images, this copy is printed in one color. Readers are directed to the electronic version of this report to view the color images; it is located at http://clu-in.org.
In Situ Treatment of Contaminated Sediments
Jon Renholds
Technology Status Report prepared for the U.S. EPA Technology Innovation Office
under a National Network of Environmental Management Studies Fellowship
Compiled June - September 1998
Figure 1 - The vessel used in the in situ chemical injection in Hamilton Harbor
--The Gander with its attached Injection Boom --

Source: http://www.oceta.on.ca/profiles/limnofix/list.html
I would like to acknowledge and thank the individuals who contributed to this paper. This includes those who provided information and those who externally reviewed and provided comments on draft documents. I would especially like to thank the U.S. EPA’s Technology Innovation Office for their immense help in completing this document.
In the past, the only option to remediate contaminated sediments was to dredge the sediment, then treat or dispose of it. Now, in situ remediation technologies are being considered for treatment more frequently. The purpose of this paper is to assess the current status of in situ treatment technologies for the remediation of contaminated sediments.
Pollutants from industry, mining, agriculture, and other sources have contaminated sediments in many surface water bodies. In the Great Lakes Region, a large area of sediment accumulation, sediment contamination poses a severe threat to human health and the environment. Efforts to clean up sediment contamination began in the 1960s; however polychlorinated biphenyls (PCBs) and p,p’dichlorodiphenyltrichloroethane (DDT) levels continued to rise in fish tissues in the 1980s, even though all sources and use of these chemicals had been banned in the Great Lakes basin since the 1970s. This rise sparked interest in the possibility that sediments were a source of toxics. Now, overwhelming evidence supports the theory that toxics trapped in sediment can adversely impact humans and the environment (White, 1998).
In September 1997, EPA completed its National Sediment Quality Survey, which was developed in response to a mandate by Congress. It is part of a three-document series titled “The Incidence and Severity of Sediment Contamination In Surface Waters of the United States,” which takes a comprehensive look at the severity of contaminated sediments in the United States. This survey uncovered that sediment contamination exists in every region and state of the country and there are 96 watersheds of probable concern. It concludes that approximately 10% of the sediment underlying surface waters in the United States is sufficiently contaminated with toxic pollutants that pose potential risks to fish, and to humans and wildlife who eat fish. One of the four goals that was established in this survey was to reduce the volume of existing contaminated sediment.
Sediment has been described as the “ultimate sink” or storage place for pollutants. Unfortunately, due to resuspension, sediment can function as both a sink and a source for contaminants in the aquatic environment (EPA, 1997). Because many toxic contaminants that are barely detectable in the water column can accumulate in sediments at much higher levels, the water column can continue to be contaminated long after the source of pollutants is controlled. Even if contaminant levels in the water column are low, adverse effects to organisms in or near the sediment can occur. Benthic organisms are easily exposed to pollutants in contaminated sediments through a variety of ways. This potentially leads to bioaccumulation of contaminants up through the food chain, which has been observed extensively in the Great Lakes (White, 1998). This effect has led to many fish consumption advisories throughout the United States (EPA, 1997).
EPA’s “Selecting Remediation Techniques for Contaminated Sediments” identified a wide array of contaminants present in sediments (EPA, 1993a). The report grouped the contaminants into eight categories:
In EPA’s 1998 National Quality Survey, the most frequent chemical indicators for the highest level of sediment contamination were PCBs, mercury, organochlorine pesticides, and PAHs, with PCBs being the most frequent. In the 50 years of production of PCBs, it is estimated that several million pounds of PCBs have entered the environment worldwide. These chemicals are very toxic and tend to bioaccumulate in fatty tissues. Therefore, PCB contamination is both widespread and damaging (Abramowicz et al, 1992).
Section 121b of the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA) and the Superfund Amendments and Reauthorization Act (SARA) establishes offsite transport and disposal of untreated contaminated materials as the least desirable alternative (EPA, 1990). Despite this, dredging and confined disposal is one of the more established and widely used ways to remediate contaminated sediments (EPA, 1994). A 1993 EPA evaluation of Superfund Records of Decision identified 49 sites where remedial actions were selected for contaminated sediment. At 30 sites, excavation and treatment were selected; at 19 sites, the remedies selected were excavation and containment (EPA, 1993b).
While dredging is often necessary for navigational reasons in many harbors, it may not be the best method for remediating contaminated sediments. Dredging can lead to the resuspension of contaminated sediments, thus contaminating the water column (EPA 1993a). In addition, transporting contaminant sediments can result in contaminant losses due to spills and volatilization (EPA, 1993a). For example, during the Hamilton Harbor project, suspended sediments appeared to flow away from the clamshell dredge (EPA, 1998). Because dredging did not remove all the contaminated sediments in the boatslip area, in situ treatment was tried as an alternative. In Zierikee Harbor located in the Netherlands, it was observed that after dredging, the top sediment layer was more contaminated than before dredging. A Dutch review identified this as a common result of dredging (Development Programme Treatment Processes, 1991).
There are four ways to remediate sediments in place:
(1) natural attenuation (natural recovery);
(2) in situ capping;
(3) waterway confinement (in situ confinement); and
(4) in situ treatment (EPA, 1994; Bokuniewicz et al, 1997).
Few in situ treatments have been conducted in the field. The major in situ treatment projects to date, which are discussed later in this paper, are listed in Table 1.
Table 1 - Selected Projects Using In Situ Treatment Methods for Contaminated Sediments
| Project Location |
Contaminants |
Treatment Type |
Date & Scale |
|---|---|---|---|
| Hamilton Harbor, Canada |
PAHs (primary), sulfides, and oils |
Biological/ Chemical |
1992/93 Pilot Scale Study |
| St. Mary’s River, Canada |
Sulfides |
Biological/ Chemical |
1991 Pilot Scale Study |
| Salem, MA (Future Project) |
PAHs |
Biological/ Chemical |
1998 Full Scale Treatment |
| Fox River, WI |
Lead |
Stabilization |
1993 Full Scale Treatment |
| Manitowoc River, WI |
PAHs and heavy metals |
Solidification |
1992/93 Pilot Scale Study |
| Hudson River, NY |
PCBs |
Biological/ Chemical |
1991 Field Research Study |
Note: An appendix lists the point of contact for each project.
In situ treatment of contaminated sediments has long been considered a possible cost-effective and ecological treatment option, but little has been done to investigate it. It encompasses a variety of methods to treat contaminated sediments without removing them from rivers, lakes, or harbors. However, because of limitations—such as saturated conditions, anaerobic environments, and ambient temperatures—the type of techniques that can be used for in situ treatment is less compared to ex situ treatment (e.g., it would be practically impossible to do a thermal treatment in situ).
In situ treatment can be divided into two areas: biological/chemical treatment methods, and solidification/stabilization treatment methods. In situ biological/chemical treatment involves the addition of microorganisms and/or chemicals to the sediments to initiate or enhance bioremediation. In situ solidification/stabilization treatment involves the addition of chemicals or cements (e.g., Portland cement and quicklime) to encapsulate contaminated sediments and/or convert them into less soluble, less mobile, or less toxic forms.
The primary advantage of in situ treatment is that the sediments are left in place, decreasing the chance of further contamination from resuspension of contaminants that are bound to the fine particles in the sediment. Another benefit is the reduction in the need for handling sediments, and the potential for exposure and consequential spills of the sediments. A third benefit is a reduction in the volatilization and irretrievable loss to the atmosphere of contaminants that are brought to the surface (EPA, 1993a). A fourth advantage is that it meets section 121b of CERCLA/SARA, which states EPA’s “preference for remedial actions where treatment permanently and significantly reduces the volume, toxicity, or mobility of hazardous substances” (EPA, 1990). A fifth advantage is that extensive long-term monitoring required for disposal facilities that handle contaminated sediments (e.g., landfills) is no longer required. A final benefit is the cost of in situ treatment.
In general, using in situ treatment appears to be less expensive than ex situ treatment or disposal of contaminated sediment. For example, Limnofix Inc. predicts that a full-scale remediation of Hamilton Harbor would cost 20% of the cost to dredge and dispose of the contaminated sediment (Murphy et al, 1995b). The 1997 “Contaminated Sediments in Ports and Waterways Report” conducted by the National Research Council estimates in situ treatment costs at $10/yd3, compared to ex situ treatment costs at $100/yd3. The Council also gives the cost for ex situ containment at $10/yd3, even though some believe that in situ treatment will cost less that ex situ containment (Bokuniewicz et al, 1997). However, while in situ treatment is generally considered a less costly alternative, EPA’s Remediation Guidance Document indicates that in situ treatment may be less cost effective than ex situ treatment because the treatment level for in situ treatment is not uniform and project goals often cannot be met (EPA, 1994).
The treatment efficiency of in situ treatment is almost always less than ex situ treatment. The lower treatment efficiency observed is due to many different factors. In the case of in situ bioremediation, an explanation for the relative inefficiency of this technology could be that only a limited amount of the contaminant is bioavailable to microorganisms, as was found during a General Electric (GE) study of PCB degradation. The 50% degradation that was observed in this study might be considered low if the bioavailability to microorganisms is not considered.
In EPA’s Remediation Guidance Document, several limitations are listed for in situ treatment. One of the more important limitations cited is the difficulty with or lack of process control. Because of variances in sediment type and contaminant distribution, it is difficult to ensure uniform dosages of treatment chemicals or to measure treatment efficiency. This can result in different levels of treatment for different areas of the sediment. Another limitation cited in this document is the impact the process has on the water column. Ideally, a remediation method will not result in the release of contaminants to the water column. However, the mixing of treatment chemicals or microorganisms may result in the resuspension of sediments or contaminants. This impact can be avoided by: using a direct injection method, as demonstrated during the Hamilton Harbor study; isolating the sediment using caissons, as demonstrated during the Fox River Study; or incorporating materials into a solid media that can be put into the sediment, as demonstrated during the microencapsulation study and the biological carpet proposal.
In the Fox River, where caissons made it possible to throughly mix sediments without concern for resuspending them, the stabilization treatment efficiency was very high (around 99.7%) (Chowdhury et al, 1996). In comparison, a sediment injection method done by Limnofix, which does not use caissons, had around 48% treatment efficiency. The injection technique used by Limnofix can never approach the treatment efficiency demonstrated in the Fox River study (Murphy et al, 1995b). However, if the mixing method can be improved, it will result in fairly large performance improvements.
The lack of natural mixing of sediment is considered by some to be the main reason why contaminants do not naturally biodegrade in the sediment. One theory is that microorganisms often have what they need to degrade contaminants, yet due to very little lateral movement of porewater in underwater sediments, the microorganisms can not get to the contaminants to degrade them (Hayes, 1998). Regardless of the reason, it appears that mixing is important to the performance of an in situ treatment process.
As mentioned earlier, the use of in situ remediation has been limited to cases where dredging is not necessary for navigational or other reasons. In theory, in situ treatment could be used to treat contaminants before they are dredged, thus preventing potential release to the environment through volatilization, resuspension, or another means. This situation was illustrated on a small scale in a Fox River project.
While in situ treatment is considered more cost effective than removal technologies, other in situ remediations, such as in situ capping or natural attenuation, are typically less expensive. The National Research Council estimates the cost of in situ capping at $1/yd3. However, in situ capping is a containment technology, not a treatment (Bokuniewicz et al, 1997).
One disadvantage of biological or chemical treatment is that the high biological oxygen demand (BOD) seen in most sediments can limit biological or chemical treatment (Bokuniewicz et al, 1997). In the Hamilton Harbor study, the presence of methane (resulting in high BOD) required that higher than expected doses of oxidant be added (Murphy et al, 1995b). The oxygen was depleted by the microorganisms because of the preferential aerobic degradation of the methane instead of the contaminants. According to John Haggard of GE’s Corporate Environmental Program, high BOD also could be responsible for the large amount of peroxide (an oxidant) used in GE’s study of PCB degradation (1998). Another disadvantage of biological or chemical treatment is that it only is applicable to organic contaminants. If metals contamination is present, the only in situ treatment options available are stabilization or solidification.
Another reason that in situ biological/chemical treatment generally has a lower treatment efficiency could be due to the poor environmental conditions (i.e., saturated, anaerobic conditions at ambient temperatures) for microbial degradation (Bishop, 1996). In order to treat the sediments in situ, these conditions must be changed. Reasons that persistent contaminants in sediments are resistant to microbial degradation include:
microorganisms
· Poor contaminant bioavailability to microorganisms
The strength of solidified sediment is important to prevent its erosion and the release of contaminants over time. Mixing conditions and curing temperature have been identified as the principal factors that influence solidified sediment strength (Kita and Kubo, 1983). Since mixing and temperature are difficult to control in situ, in situ solidification may be more limited than other in situ treatments. In addition, in situ solidification may not change the toxicity of the contaminants in the sediment. Therefore, long term performance is a concern because erosion and diffusion could eventually release the contaminants (EPA, 1994).
Table 2 - Main Advantages and Disadvantages of In Situ Treatment
| Advantages |
Disadvantages |
|---|---|
| Relatively inexpensive |
Lack of process control |
| Usually results in less resuspension of contaminated sediments than removal technologies |
Poor environmental conditions for treatment |
| Treats, does not contain, contaminants |
Lower treatment efficiency than ex situ treatment |
| Reduces handling and exposure of sediments |
Limited experience with in situ treatments |
Biological/chemical treatment uses microorganisms and/or chemicals to degrade toxic contaminants in the environment. Although many contaminants can be degraded by naturally- occurring microorganisms, these microorganisms often degrade the contaminants too slowly. Therefore, the addition of microorganisms and/or chemicals is used to stimulate the degradation of contaminants.
TREATMENT PROJECTS - IN SITU BIOLOGICAL/CHEMICAL
Few pilot- or full-scale projects using in situ biological/chemical treatment for remediating contaminated sediments have been conducted to date. However, lessons learned from these projects can be applied to future in situ treatment applications. In addition, bench-scale and laboratory-scale studies can provide insight into the direction of research and applications of in situ treatment and what will work for future projects. This section presents six different treatment projects: Hamilton Harbor (Dofasco Boatslip), Canada; St. Mary’s River, Canada; Salem, MA (Future Project); In Situ Hudson River Research Study; Microencapsulation Study; and Biological Carpet Proposal. These projects include pilot studies, a field research study, a laboratory study, and a proposal for a future study.
Hamilton Harbor (Dofasco Boatslip), Canada
Introduction
Hamilton Harbor, which is located on Lake Ontario in Canada, was identified by the International Joint Commission as one of 43 Areas of Concern in the Great Lakes Region because of environmental impairments (Irvine et al, 1997). The harbor is contaminated with metals, sulfides, oils, and a variety of organic compounds, especially PAHs. Most of the contamination resulted from historic industrial waste streams, but current sources, such as sewer overflows, atmospheric inputs, and coal pile runoff, continue to contaminate the harbor.
Simple projections of the costs to remediate the harbor indicate that dredging and disposal of all hot spots would cost $20 million, while similar cleanup of the rest of the contaminated sediments would cost $4 billion. After examining these projected costs, the National Water Research Institute of the Environment Canada decided to conduct several pilot-scale studies to remediate the contaminated sediments in Hamilton Harbor, which included an in situ treatment study conducted from 1992 to 1993 in an area of the harbor called Dofasco Boatslip (Murphy et al, 1995b).
Dofasco Boatslip is 1 km long by 100 m wide. In 1988, about 3000 m3 of sediment was dredged at a cost of $600,000. The dredging was not that successful because the silty sediment flowed away from the clamshell dredge head, resulting in a mix of clean and contaminated sediments. The dredging and ship traffic removed most of the contaminated sediments, but several hot spots of contamination remained.
Treatment Technique
The objective of the in situ treatment in the Dofasco Boatslip area was to stimulate anaerobic bioremediation with the natural microorganisms in the sediment using chemical injection of oxidants and/or nutrients. After characterization of the contaminated sediment, a laboratory study to examine the feasibility of applying the treatment to the field was conducted to determine the optimal conditions for bioremediation. The sediment samples for the laboratory study were kept anoxic, and calcium nitrate and an organic amendment were mixed with the sediment. The laboratory study yielded positive results, prompting Environment Canada to proceed with the pilot-scale in situ chemical injection.
The Department of Fisheries and Ocean vessel, the Gander, was fitted with an 8 m wide injection boom to inject the chemicals into the sediment. The boat was 8.2 m long, 3 m wide, had a draft of 0.4 m, and could be loaded with 6 tons of calcium nitrate (see Figure 2). During the first three treatments, only farm grade calcium nitrate was injected into the sediments: 3.6 tons on July 28, 1992; 3.89 tons on September 15-17, 1992; and 6 tons on April 27, 1993. On September 22, 1993, five tons of calcium nitrate along with five tons of organic amendment were injected again. Samples of the sediment were taken to determine the concentration of total petroleum hydrocarbons, sulfides, and PAHs before and after the treatments were performed. The air was monitored for the release of volatile organics for health and safety reasons, and sediment resuspension was measured during the injections (Murphy et al, 1995b).
Figure 2 - In Situ Chemical Injection Method Used at Hamilton Harbor (Dofasco Boatslip)
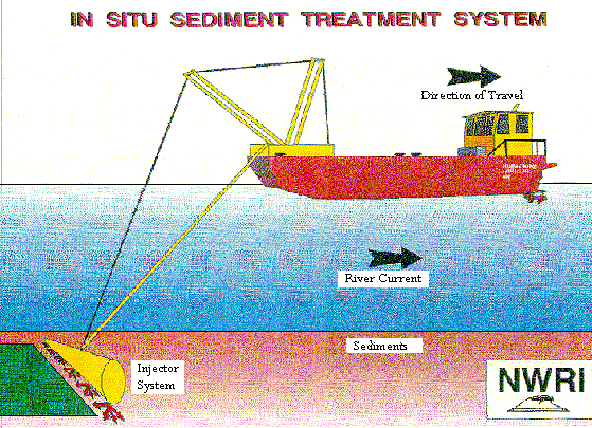
Source: http://www.oceta.on.ca/profiles/limnofix/list.html
Cost and Performance
The laboratory studies on the sediments from Dofasco Boatslip indicated that the microorganisms biodegraded approximately 78% of the oils and 68% of the PAHs in 197 days (Murphy et al, 1995b). Despite the fact that the sediment used for the laboratory studies was some of the most contaminated [up to 2000 micrograms per gram (µg/g) of PAHs], the high- molecular-weight PAHs degraded just as quickly as the low-molecular-weight PAHs.
The 1992 chemical injection in Dofasco Boatslip was fairly successful at degrading some organic compounds—for example, 80% of the toluene and 86% of the ethylbenzene—but there were problems with the PAH degradation. Only 15% of the PAHs were degraded in the 1992 chemical injection, and naphthalene concentrations increased by 195%. However, since the naphthalene is the intermediate compound during the biodegradation process, its concentration decreased below the original pretreatment levels during the 1993 injections. After the 1993 injections, the total petroleum hydrocarbons (TPHs) were reduced by about 57% and, in the flat areas, the total PAHs were reduced by about 48%.
Monitoring during all the injections indicated that both the release of volatile organics and the resuspension of sediments were minimal and not of concern. Nearly all of the free hydrogen sulfide was degraded, but the acid volatile sulfides appeared to be resistant to oxidation by calcium nitrate (Murphy, 1995b). The treatment efficiency was less in areas with steep slopes and areas with logs because the injection equipment tended to jump or slip in these areas. Although the injection equipment shuddered when it hit a log, it was not damaged and treatment was not delayed.
When the chemical injection was performed, diffusion of nitrate into deeper sediments caused methane to be released to the surface, where it competed with the contaminant for the nitrate oxidant. Therefore, to increase PAH degradation, a higher dose of nitrate (or another oxidant) would be required to overcome its use by in fluxing methane. It was promising that the addition of organic amendments stimulated bioremediation. Continued study was planned to determine the benefit of increasing the injection of organic amendments, but treatment in Dofasco Boatslip was stopped because of continued contamination of the sediments from coal pile runoff. When contaminated sediment began to accumulate on top of the treated sediment, it became difficult to determine treatment efficiency (Murphy, 1998). Environment Canada has acquired a patent for the treatment method, and has licensed the technology to Limnofix Inc., which is working to improve the injection equipment to improve the treatment efficiency (Babin, 1998).
A large portion of the treatment costs was associated with extensive monitoring; however, such monitoring may not be required in a full-scale study. After conducting this study, the National Water Research Institute predicted that it should be possible to bioremediate sediments for about 20% of the cost of dredging and storage in a confined disposal facility (Murphy et al, 1995b). The Institute did not provide the exact costs for the study, but investigators estimate that between 15% to 30% of the total treatment costs were chemical costs (Babin, 1998).
Introduction
Prior to the treatment in Hamilton Harbor, Environment Canada conducted a pilot-scale study of in situ chemical injection to stabilize sulfide in sediments. This study was conducted in St. Mary’s River (Murphy et al, 1995a), which connects Lake Superior and Lake Huron and is contaminated with a large amount of organic waste (including various forms of sulfur chemicals from an Algoma Steel Mill, poorly treated sewage, and a large quantity of wood fiber) (Murphy et al, 1995a). The sediment in the river contains approximately 99% wood fiber and 1% silty sediment (Babin, 1998).
Prior to treatment, it was believed that the sulfide toxicity greatly reduced the natural bioremediation of the organic contamination. Thus, treating the sulfide toxicity with iron, which reacts rapidly with hydrogen sulfide to stabilize it, could greatly improve the condition of the sediment.
The same equipment used in the Hamilton Harbor project—the Gander equipped with an injection boom—was used for this study. Sediment resuspension was measured with sediment traps during the injection treatments. It was determined that although a pressure wave proceeded the injection bar, the injection resulted in little suspension of the woody sediment. As a result, resuspension was determined not to be a problem during this project.
Procedure
The treatment involved injecting ferric chloride into the sediments to inactivate reactive sulfide and reduce sediment toxicity. On July 10, 1991, 600 L of ferric chloride was injected in an area measuring 90 m by 12 m. On October 6, 1991, a second ferric chloride injection was done in a different area measuring 200 m by 36 m. In the first injection, only the top 9 cm of the sediment was treated, which resulted in a decrease of 81% in the concentration of reactive sulfide and acute toxicity. In the second injection, modifications were made to the injection bar to enable treatment of the top 15 cm of the sediment, which resulted in a 57% decrease in the concentration of reactive sulfide and acute toxicity. Although it could not be determined what effect the treatment had on the toxicity and the natural biodegradation of the other organic contaminants in the river, it is believed that the treatments added about one year of oxygen diffusion to the sediment. (Murphy, 1995a).
In the fall of 1998, Limnofix Inc. is planning to conduct a full-scale treatment of intertidal zone sediments to enhance the biodegradation of organic contaminants. The treatment site, which is located in Salem, Massachusetts, is an intertidal zone of approximately 2,000 square yards where there is PAH contamination (mainly naphthalene) from an abandoned manufactured gas plant. At the site, there are eight to ten inches of contaminated sand that is underlain by clay. The contamination is slowly migrating through the intertidal zone above the clay layer.
The bench-scale and pilot-scale studies have indicated that oxidant limited the biodegradation at the site. The treatment will involve injection of calcium nitrate to stimulate biodegradation of the PAHs, much like what was conducted at Hamilton Harbor. Because the site is in an intertidal zone, the treatment is being conducted during low tide with a special tractor that will pull a modified harrow to inject the calcium nitrate (Babin, 1998). Although this treatment study will provide no further information on the underwater injection conducted in Hamilton Harbor, the results should help shed light on whether the biodegradation efficiency of PAHs can be improved.
Introduction
From 1946 to 1977, two GE plants located in Fort Edwards and Hudson Falls, New York, caused extensive PCB contamination of the upper Hudson River (Abramowicz, 1992). In 1991, GE conducted the first extensive study of the natural and accelerated biodegradation of these chemicals. From previous laboratory studies, GE determined that PCBs could be anaerobically dechlorinated to monochlorinated or dichlorinated biphenyls. The monochlorinated or dichlorinated biphenyls then could be completely biodegraded aerobically by a small number of microorganisms.
For this study, GE selected a site in the Hudson River near the Town of Moreau, New York, where extensive dechlorination already had occurred, to try to aerobically biodegrade lower- chlorinated biphenyls. The site is located about two miles downstream from the GE plant in Fort Edwards and the study was conducted from August 9 to October 21, 1991.
The field study consisted of driving six caissons eight feet into the sediment to form test cells, which were six feet in diameter and a half-inch thick (see Figure 3). Measures were taken to ensure that there were no leaks in or out of these test cells. Of the six test cells, two were used as controls [a high-mix cell (C1) and a low-mix cell (C4)], two were used as duplicate low-mix treatments with additions of oxygen, nitrogen, and phosphate (C5 and C6), and two included a microorganism known to biodegrade lower chlorinated PCBs (H850), as well as oxygen, nitrogen, and phosphate—one high-mix cell (C2) and one low-mix cell (C3).
The two high-mix test cells (C1 and C2) were mixed with an impeller that rotated at 40 rpms to resuspend a large amount of sediment. The four low-mix test cells (C3, C4, C5, and C6) were mixed with a plow-like rake that rotated at three rpms to turn over the sediments without resuspending a large amount of them. Oxygen in the sediments was maintained by automatically adding peroxide when the oxygen fell below a predetermined level. Oxygen levels were kept below saturation levels to prevent losses to the atmosphere and to enable monitoring of the total oxygen consumed. Nitrogen and phosphate were added to the test cells at regular intervals; biphenyl also was added at lesser intervals to study its effect on triggering aerobic PCB degradation (Abramowicz et al, 1992).
Figure 3 - GE’s Caisson Configuration
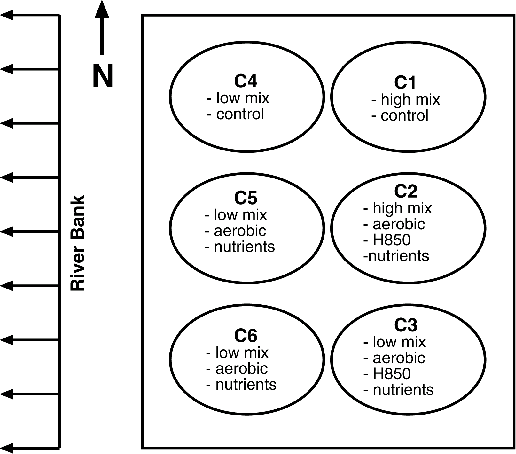
Performance
Although there were difficulties with measuring the biodegradation rate of PCBs during this 10 week test, there was evidence that accelerated PCB degradation did occur. Besides the consumption of the oxygen and nutrients (nitrogen and phosphate) that were added to the test cells, there was a temporary appearance of chlorobenzoates during the treatment. From previous laboratory studies, chlorobenzoates were determined to be an intermediate in aerobic biodegradation between lower-chlorinated PCBs and complete mineralization to carbon dioxide, water, and chloride ions. The temporary appearance of these compounds indicated active biodegradation.
PCB degradation in the cells was measured using three methods. The results are listed in Table 3. The original analysis of PCB degradation was based on the measured average PCB concentrations, which indicated that the low-mix treatment cells without microorganisms (C5 and C6) had higher rates of PCB degradation (72.6% and 68.5%, respectively) than the high-mix treatment cell with inoculation (C1) (41%) (refer to Table 3). However, these measurements were an inaccurate indication of the actual PCB degradation that occurred. Therefore, PCB losses also were measured with two other methods. The second method correlated the amount of PCB with the total organic carbon (TOC) and then measured the TOC content to get the PCB losses. This method can be done because hydrophobic contaminants such as PCBs typically reside and adhere to the organic fraction of the sediments. The third method was used to normalize the data using a non-degradable PCB as an internal standard. Although there were no PCBs that were completely non-biodegradable, one of higher molecular weight PCBs was selected (called peak 61). Therefore, the results are considered conservative.
Table 3 - Estimated PCB Losses During GE’s Hudson River Treatment Study
| Caisson |
Method 1 Average PCB Concentration |
Method 2 Average PCB/TOC |
Method 3 Peak 61 PCB Normal |
|---|---|---|---|
| C1 |
+ 8.7% * |
- 30.7% * |
- 14.4% |
| C2 |
- 41.0% |
- 44.7% |
- 42.4% |
| C3 |
- 36.8% |
- 55.5% |
- 37.8% |
| C4 |
- 41.8% |
+ 8.4% * |
- 4.3% * |
| C5 |
- 72.6% |
- 53.0% |
- 40.5% |
| C6 |
- 68.5% |
- 46.0% |
- 38.7% |
Note:Negative values are losses and * indicates that the changes are not statistically significant at a 95% confidence level.
Source: GE Research and Development, Abramowicz et al, 1992
After comparing the two different measurements, GE determined that between 38% and 55% of the PCBs were degraded in the test cells, there was a minimal amount of degradation in the high- mix control cell (C1), and no significant change in the low-mix control cell (C4) (Abramowicz et al, 1992). Little difference was noticed between the treatment cells that received microorganism inoculation and those that did not. Therefore, it was concluded that the microorganisms needed to aerobically biodegrade the lower-chlorinated PCB were naturally present in the sediment. In addition, little difference was observed between the low-mix and high-mix treatment cells.
Conclusions
Low-mix treatment might be more applicable to an in situ treatment, assuming that it would have significantly less resuspension of sediment. However, the resuspension of sediment due to mixing in the test cells was not evaluated in this study. Also, the sediment was “ground up” in the low mix caissons as a result of the mixing method; therefore, a new method of mixing would have to be employed for an in situ treatment (Haggard, 1998). PCB degradation efficiency is believed to be significantly greater at higher PCB concentrations because more would be bioavailable to degrade. The PCBs that were not degraded in this study were bound tightly to the sediment and were therefore not bioavailable to microorganisms during the duration of the study. Contaminants not bioavailable to the microorganisms likely are not bioavailable to other organisms as well, but due to slow desorption of the contaminant over time, the PCBs not degraded in this study could become bioavailable at a later date (Haggard, 1998).
Although this study did not show how an in situ treatment technique would work, it did demonstrate that PCBs can be actively destroyed in situ by a sequential anaerobic then aerobic biodegradation process. There are varying degrees of optimism about microbially-degrading PCBs, but there has been no follow-up studies of this scale to date. Due to the low treatment efficiencies seen in a controlled environment, the general opinion is that PCB bioremediation works, but it doesn’t work that well (Cowgill, 1998). Even though the project was an important scientific study, it was not a feasibility study and the engineering of applying what GE did to an actual in situ treatment could be difficult (Haggard, 1998).
In 1992, Technology Resources Inc., SBP Technologies Inc., and the U.S. EPA Environmental Research Laboratory in Gulf Breeze, Florida, evaluated a delivery technology for microorganisms and nutrients to sediments. The idea was to microencapsulate microorganisms and additives, such as nutrients, in order to enhance bioremediation in slurry reactors (ex situ treatment of sediments or soil) or in-place sediments (Lin et al, 1992).
To test this approach, two microorganisms (a fluoranthene and a phenanthrene degrader) were encapsulated in a polyvinyl alcohol matrix (PVA) and then freeze dried. Studies showed that the encapsulation process resulted in little loss in the viability of the microorganisms. The encapsulated phenanthrene degrader was tested for phenanthrene biodegradation in soil slurry and packed soil in flasks. In the soil slurry, the capsules dissolved in 30 minutes and the biodegradation of the phenanthrene was essentially complete within 33 hours. However, in the packed soil, the capsules did not dissolve in a moisture content of approximately 20% (Lin et al, 1992).
Although this was only a laboratory study, it illustrated the potential for microencapsulation as an innovative method to deliver microorganisms and nutrients to treat sediments in situ. Further study is planned to examine potential enhancement of the biodegradation process by improving the encapsulation process as well as including nutrients with the microencapsulation.
An innovative proposal to treat contaminated sediments in situ is the use of a “biological carpet,” which was developed by Michael A. Heitkamp and William P. Stewart of the Monsanto Company. This “carpet,” which is a non-biodegradable porous nylon biocarrier made from melted wet nylon that is inoculated with a variety of chemicals, compounds, or microorganisms, is used to treat liquid-waste streams. It can be inexpensively produced from nylon manufacturing wastes and recycled nylon sources, such as used carpets.
Because the biological carpet’s pore sizes are larger than conventional biocarriers used in the chemical industry, it can be inoculated with a variety of items, including microorganisms. Laboratory studies have indicated that most of the inoculated material stays in the nylon. Therefore, research is being conducted to inoculate the carpet with activated carbon to absorb contaminants (Heitkamp and Stewart, 1996).
Hap Pritchard from the Naval Sciences Research Facility is pursuing the idea of using the biological carpet for in situ treatment of sediments by attaching it to a fabric, such as a geotextile, and laying it down with the nylon protruding into the sediment, much like an upside down carpet. A variety of items would then be inoculated into the nylon—such as nutrients, microorganisms, or activated carbon—to either absorb or bioremediate contaminants in the sediment. The “carpet” could either be removed at a later date, or, because it does not biodegrade, it could be capped and left for an extended period of time. Due to lack of funding, no studies have been conducted to date to investigate this approach (1998).
IN SITU SOLIDIFICATION/STABILIZATION TREATMENT
In situ solidification/stabilization detoxifies contaminants in the sediment either by physically isolating the sediments in a solid form and/or by stabilizing the contaminants by changing them into a chemically-unavailable form. While it is one of the few techniques available to treat metals in situ, it is generally not used to treat organics due to their tendency to be more unstable and capable of degradation. Solidification and stabilization techniques have been used for some time to treat sediments ex situ, but only recently have they been applied in situ.
Solidification reduces the amount of sediment churned up due to disturbances. The contact area of sediment particles with overlying water is also reduced, thus decreasing the potential for contaminant release (Kita and Kubo, 1983). Furthermore, the adsorption capacity of solidified sediment is increased due to the increase in the inner surface area after adding cement (Shimoda, 1992).
Cement and lime are generally used as solidificants; therefore solidified sediments generally have high pH values. This results in a prevention of a chemical release. Under alkaline conditions, heavy metals generally form hydroxides, which have a low solubility (Kita and Kubo, 1983; Wada, 1992; Shimoda, 1992; Meyers et al, 1994). In a study conducted by Bethel Inc., sediments that were treated with lime caused stabilization of 80% of all the metals (Shimoda, 1992). However, sediment chemistry is complicated. A few metals form complex ions in high pH conditions and become more soluble. In addition, sediments are generally found in reductive conditions underwater (due to high BOD sediments that typically are not oxidized) and contain large amounts of sulfur compounds. In this environment, metals are in the form of sulfides, which have solubilities that are slightly lower than those of hydroxides. Mercury compounds, which are extremely stable as sulfides, have very unstable hydroxides. Therefore, despite the stabilizing effects of hydroxide formation, adding cements in these cases may increase metal solubility (Kita and Kubo, 1983).
TREATMENT PROJECTS - IN SITU SOLIDIFICATION/STABILIZATION
Five in situ solidification/stabilization projects will be discussed in this section: a pilot study on in situ lead fixation in Wisconsin’s Fox River; a research project involving Wisconsin’s Manitowoc River; Japanese solidification activities; a laboratory study conducted by the U.S. Army Corps of Engineers; and several laboratory studies on in situ metal fixation.
Introduction
During a Wisconsin Department of Transportation (WDOT) project to dredge sediment for reconstruction of an historic bridge over the Fox River near Menasha, Wisconsin, the sediment was found to be contaminated with lead, partially from lead paint chips from the bridge as well as from unknown sources. Because this sediment was toxic, as determined by EPA’s Toxicity Characteristic Leaching Procedure (TCLP), the Resource Conservation and Recovery Act (RCRA) requires that it: 1) be treated as a hazardous waste if it is removed and transported; or 2) be treated as a nonhazardous waste if it is treated in situ before removal and no longer leaches as determined by a TCLP test.
Because of the complications and cost of dealing with removal of contaminated sediments, the WDOT hired RMT Inc. to design an in situ underwater lead treatment system to fix the lead to the sediment (Chowdhury et al, 1996). In May 1993, RMT Inc. conducted this treatment.
First, cofferdams were driven into the bedrock to a total depth of 15 feet (the water depth ranged from seven to eight feet in the treatment area) and sealed. Water was then pumped out of the cofferdams to maintain an inward gradient, as is also done for normal bridge construction without contamination. Throughout the treatment, the water inside the cofferdams was kept at approximately three feet below the water surface level of the river by pumping continuously at about 50 gallons per minute (gpm). This pumped water, which was considered to be potentially polluted, was then pumped to the local wastewater treatment plant.
Next, a mixture of chemical additives was prepared, which included fertilizer grade phosphate, magnesium oxide, and a reactive form of limestone, into the sediment. Phosphate was added because it quickly reacts with lead to form a compound that is highly resistive to leaching over a wide range of pH values. Limestone was added to enhance the chemical reaction, to help dewater the sediment, and to reduce the soluble phosphate concentration in the water by binding it up. Magnesium oxide was added to buffer the high pH levels (due to the limestone) during the treatment.
These chemical additives were added and mixed in three different lifts with a clamshell dredge. About half of the chemicals were added in the first lift, a third in the second lift, and the remaining sixth in the third lift. After each lift was properly mixed and adequate time was allowed for the reaction, the stabilized sediment was dredged and put into a containment basin, from which porewater was allowed to drain. This porewater was sent to the wastewater treatment plant for treatment and the stabilized sediment was sent to a landfill as a nonhazardous waste (see Figure 4). During the mixing process, much of the sediment was suspended in the water column. Before the pumping was stopped and the cofferdams removed, the suspended sediment was allowed to settle in order to avoid further contamination of the river.
Figure 4 - In Situ Lead Treatment in Fox River
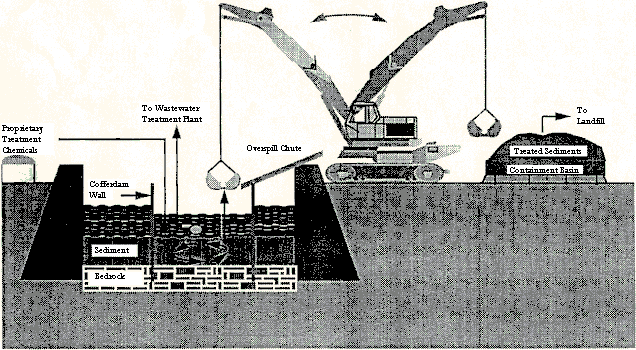 Source: Chowdhury et al, 1996
Source: Chowdhury et al, 1996 Performance
According to a TCLP test conducted after the treatment, the leachable lead was reduced to less than 0.26% of the highest observed untreated value—in other words, better than a 99.7% treatment efficiency was observed. The leachable lead also was well below the hazardous limit level and was expected to remain there because the phosphate-lead composite was considered stable over a wide range of environmental conditions. This finding led to little concern about environmental damage resulting from the landfilling of the stabilized dredged sediment (Chowdhury et al, 1996).
Cost
Five-hundred tons of sediment were treated and dredged during this treatment. The total cost for the treatment was $132,000, most of which paid for preparation (e.g., pretreatment studies and permitting) of the sediment. It was estimated that the actual project was only $30,000 to $40,000 (Warner, 1998). Because RMT Inc. was able to treat the sediment in situ, WDOT avoided dealing with the sediment as a hazardous waste, which would have cost three to four times the amount of treating it in situ. This resulted in a savings of around $100,000; a significant amount of money for such a relatively small-scale treatment. Because a large excess of chemicals were used to ensure complete treatment, a larger-scale project could result in substantial savings by reducing the amount of chemicals added per ton of sediment.
Conclusions
Because the water was completely turbid during treatment, investigators concluded that it would have been impossible to treat the sediment without the cofferdams (Warner, 1998). This could present problems in larger-scale treatments where use of cofferdams to section off the contaminated portion is not feasible. Despite this limitation, treatment within the cofferdams saved a considerable amount of money, and the technique could be applied to similar contaminated sites. Because of the high treatment efficiency that was observed in the Fox River, RMT Inc. acquired a variety of patents for this treatment process.
In Situ Solidification - Manitowoc River, WI
Introduction
An area of Wisconsin’s Manitowoc River was contaminated with PAHs and several heavy metals from a coal gasification plant that operated throughout the mid-1900s, before regulations eliminated the source of contamination. The responsible party for contamination from the plant, a local electric company, hired Mill-Guard Environmental Corp. to examine the possibility of using in situ solidification to treat the contaminated sediments. The site selected for the demonstration had a water depth of approximately six meters.
The in situ solidification method involved the use of a 6-foot diameter steel cylinder to case a hollow-stemmed Kelly bar, which was used as a mixer and slurry injector (similar to the process seen in Figure 5). The 1.8 m by 7.6 m steel cylinder was lowered 1.5 m into the sediment, and a Portland cement/fly ash slurry was injected into the sediment in an attempt to solidify it (EPA, 1994). Approximately 400 lbs of cement slurry were mixed for every cubic yard of sediment.
Performance
Six different 6-foot diameter areas of sediment were treated: three in the winter of 1992, and three in the winter of 1993. Unfortunately, a number of different problems arose with the treatments. Initiating the mixing resulted in a release of sediment porewater, which contained significant oil and aqueous phase contaminants, to the overlying water column. Also, due to the addition of mass, the sediments rose three to four feet up the cylinder, causing them to be only semi-solidified (probably due to the dilution of the cement by the surrounding water). In the 1992 treatments, the level of the water in the cylinder rose six feet above the level of the water surface in the river, which resulted in major sediment resuspension that took some time to settle. To resolve this, a bladder was installed above the sediments in the 1993 treatments. However, this resulted in a large increase in pressure that caused some blow out of sediments from the bottom of the cylinder during mixing (Fitzpatrick, 1998).
Figure 5: In Situ Solidification Treatment using a caisson.
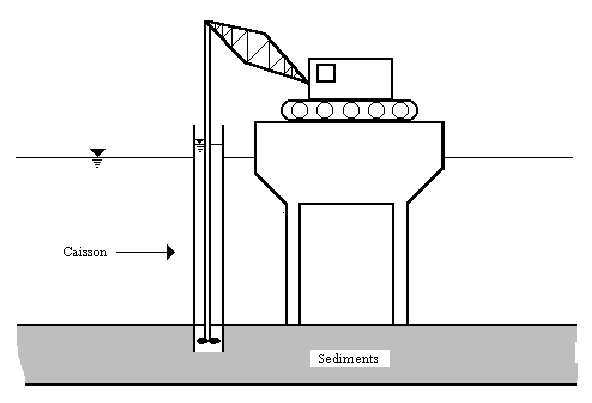
Source: Remediation Guidance Document, EPA, 1994.
Conclusions
The Wisconsin Department of Natural Resources (WDNR) determined that the in situ solidification process did little to treat the sediments. Therefore, no further treatments were conducted. A major reason for the failure could be that the mass balances of the addition of the cement/fly ash were not thoroughly considered (Fitzpatrick, 1998). Another reason for the failure could be the inability to control the mixing conditions and curing temperatures during the process (Kita and Kubo, 1983). There was no follow-up study on the sediments that were semi- solidified; therefore it can not be determined if the treatment might have been slightly effective at decreasing contaminant leaching.
Contaminated sediments have long been a problem in Japan. Back in the 1970s, Japan began to try different approaches to clean up contaminated sediments using different remediation methods, including dredging, capping, and solidification. Most of this work was ex situ solidification of dredged material, but in situ stabilization always has been considered a possibility (Wada, 1992). Japan has conducted a number of laboratory studies using Portland cement and/or lime with or without various additives to test how solidification isolates sediments (Kita and Kubo, 1983), but no demonstrations have been conducted.
At the 7th U.S./Japan Experts Meeting on the Management of Contaminated Sediments, four methods to apply solidification were discussed:
Although an actual in situ stabilization has not been demonstrated in Japan, a dewatering project that involved in situ stabilization methods was conducted in the Hama River. Caissons were put in the Hama River to isolate the area to be dewatered in order to solidify the sediments in place. Between 1974 and 1976, the 40 m wide river was cut in half by a double steel sheet piling. The sediments were solidified with 100 kg/m³ of ordinary Portland cement by a ship-type treatment machine. Part of the 35,000 m³ of solidified sediment was removed and part was left in place to improve the bed sediments after the water level was restored (Kita and Kubo, 1983).
U.S. Army Corps of Engineers Laboratory Study on Chemical Mobility After Solidification
In a laboratory study, the U.S. Army Corp of Engineers examined chemical mobility after solidification of sediment using ordinary Portland cement. Sediments from Everett Harbor, Washington; Indiana Harbor, Indiana; Buffalo River, New York; and New Bedford Harbor, Massachusetts, were analyzed. Each had slightly different reactions to the treatment, but similarities were observed. Some metals’ leachability, like arsenic and cadmium, were completely eliminated by the treatment. Yet for all four sediments types, complete metal stabilization was not achieved, and in some cases, metal mobility was enhanced (Meyers et al, 1994). This study only looked at chemical stabilization. Therefore, it was concluded that while chemical stabilization has to be examined on a case-by-case basis, physical stabilization is an important factor not dependent on the type of contamination or sediment.
Several Laboratory Studies on In Situ Metal Fixation
The stabilization of nutrients, such as nitrogen and phosphorus, has been practiced for some time as a solution to eutrophication problems. In these cases, alum or other types of nutrient fixers are added to sediments to stabilize the nutrients and make them unavailable to algae (EPA, 1994). Results have been fairly successful. In situ stabilization of other chemicals, such as metals, is still in the research stage, although in situ stabilization of lead has been conducted in Wisconsin’s Fox River.
In 1989, a laboratory study was conducted in Albany, Western Australia, to investigate the fixation of mercury contamination to sediments from Prince Royal Harbor using zero valent iron. At the time of the study, Prince Royal Harbor had high concentrations of mercury in the water column, especially after storms, from the resuspension of mercury in the sediments.
The primary reaction used in this study converts ionic mercury to its reduced insoluble metallic state using zero valent iron. A secondary reaction stabilizes methyl mercury (a highly toxic compound due to its high affinity for iron hydroxides). The addition of zero valent iron removed the majority of the mercury from the water column within one minute. However, a side reaction produced iron hydroxides from the zero valent iron, which formed a red scum on the water surface. In a follow-up experiment, a thin sand cap was added on top of the iron, which resulted in the same treatment without visual discoloration of the water. It was concluded that this was a feasible method to fix mercury in an insoluble form (Schulz, 1989).
Another laboratory study conducted in 1990 examined the use of aluminum salt to stabilize cadmium in sediment. In this study, sediment inoculated with cadmium, as well as contaminated sediment from Onondaga Lake, New York, were used. Various amounts of aluminum nitrate were mixed with the sediment at a pH of 9.5. To test the leachability of the stabilized contaminated sediment, both a nitric acid extraction at pH 5.0 and an ammonium acetate extraction at pH 8.0 were used. It was found that at a concentration of 0.2 mM Al/m2, about 60% and 90% of the cadmium was retained from the acid and acetate extractions, respectively. It was postulated that the acetate extraction closely replicated actual bioavailability of the cadmium; therefore, 90% treatment of the cadmium was fairly promising (Letterman and Meng, 1990). However, if the aluminum nitrate only can be added to the sediment at a pH of 9.5, this stabilization treatment would be difficult to apply in the field.
PLANNED/ONGOING ACTIVITIES FOR IN SITU TREATMENT
There are many different studies of in situ treatment currently on going, but most are laboratory studies at the academic level. The University of Washington and the University of Waterloo are looking at treating PAHs and PCBs, respectively. Also, EPA’s National Risk Management Research Laboratory (NRMRL) is researching possible strategies of in situ biorestoration and natural attenuation.
The University of Washington is conducting research on PAH anaerobic degradation in marine environments. To date, nearly all in situ treatments have been performed in freshwater, and contaminated marine sediments have not been evaluated (Bokuniewicz et al, 1997). Stuart Strand, University of Washington, and others have found that lower weight PAHs can be degraded by nitrate-reducing marine bacteria, as well as by sulfate-reducing cultures and that methane addition increases PAH degradation. They believe that the addition of nitrate will increase natural rates of biodegradation in sediments and under sediment caps. This research has been funded by the National Oceanic and Atmospheric Administration (NOAA) through the Washington Sea Grant Program, but University of Washington researchers are looking for more funding to study the increase in biodegradation rates of PAHs in marine sediments with addition of nitrate.
Researchers at the University of Waterloo have found that methane additions can stimulate anaerobic PCB biodegradation. Similar to the study done by GE, they are investigating sequential anaerobic then aerobic degradation of PCBs. In studies to date, researchers have found that biodegradation of PCBs at concentrations above 100 parts per million (ppm) is independent of concentration and fairly rapid, while under 50 ppm it is minimal and linear. This confirms GE’s assessment that the bioavailability of PCBs is a important factor to consider, but further study is needed. The Waterloo researchers believe that bioremediation is a slow process but is feasible for in situ treatment approaches. They have submitted proposals to conduct a study of a river model that will examine PCB degradation with the addition of MeOH (a source of methanol). However, they are still waiting for funding. They also believe that an in situ treatment, like the one conducted in Hamilton Harbor, could be performed using a barge equipped with injection capabilities, while manipulating anaerobic then aerobic conditions necessary for PCB degradation (Lee, 1997).
While EPA’s NRMRL is mostly looking at natural attenuation of contaminated sediments, they also have developed an in situ treatment method using hollow membrane fibers and silica gel beads with encapsulated nutrients (Tabak and Bishop, 1998). The idea behind the hollow membrane filters is to inexpensively transfer gases, such as oxygen, to the sediments (Bishop, 1998). NRMRL is currently looking at bench-scale reactor systems and, after two years, are planning to do a pilot or field implementation by laying down several hundred membrane filters into contaminated sediment cores obtained from a selected site (Tabak and Bishop, 1998).
In recent years, several formal coalitions have started to evaluate technologies to remediate contaminated sediments. EPA’s Superfund Innovative Technology Evaluation Program (SITE) has designated contaminated sediments as one of their environmental emphasis areas. The program is interested in all remediations of sediments including in situ treatment (for more information on the SITE program, visit its web page at http://www.epa.gov/ord/SITE). The Remediation Technology Development Forum (RTDF) formed the Sediments Remediation Action Team in March 1996, which established an In Situ Treatment/Confined Disposal Facilities Subgroup. This Subgroup will examine the feasibility of in situ treatment (for more information on the RTDF, visit its web page at http://www.rtdf.org).
There continues to be some interest with in situ treatment of contaminated sediments, but field studies currently are not being conducted, primarily due to the lack of funding. This is observed even with innovative treatment proposals, such as the biological carpet treatment. Field studies are limited due to the general reluctance of project managers and responsible parties to use innovative treatments, because it is easier to pay a little more to get the assurance of an established technology. Additionally, because there is a shortage of research dollars, it is speculated that current funding is being directed at shorter-term projects where the results will be seen sooner (Timberlake, 1998). This could reflect in the lack of funding observed for in situ treatment techniques.
Although there have been a limited number of pilot- and full-scale in situ treatment applications, several conclusions can be drawn. The in situ treatment in Hamilton Harbor and GE’s Hudson River field study both resulted in approximately 50% treatment efficiencies. These are very low compared to ex situ treatment, but it is unlikely that in situ treatment will ever reach the treatment efficiencies of ex situ treatments, such as wet air oxidation thermal destruction, which can attain treatment efficiencies of up to 99% for PAHs (EPA, 1994). The low-treatment efficiency of in situ treatment techniques is most likely due to the bioavailability of the contaminants and the lack of process control.
The bioavailability limitation, as was seen in both GE’s study and in the laboratory studies completed by the University of Waterloo, occurs because the organic contaminants often are tightly bound to the silt fraction of the sediment, resulting in an inability of the microorganisms to degrade them. A possible advantage of this is that contaminants that are not bioavailable to microorganisms are not available to benthic or other organisms either (Haggard, 1998).
The lack of process control is the primary limitation observed with in situ treatment approaches (EPA, 1994). From the pilot studies conducted so far, the biggest difficulty appears to be the inability to ensure uniform dosages and to properly mix the treatment chemicals into the sediments while keeping them in place. An example of the lack of process control was illustrated during the in situ solidification demonstration in Manitowoc River. In this unsuccessful demonstration study, Mill Guard Environmental Corp. could not control the mixing of the cement/fly ash slurry with the sediment (Fitzpatrick, 1998). However, process control was achieved in RMT Inc.’s lead stabilization project in the Fox River, where a treatment efficiency of over 99% resulted by isolating a section of the river with caissons to enable adequate mixing of treatment chemicals with the sediment (Warner, 1998).
Looking at the relatively high treatment efficiencies that are observed in most laboratory studies, it seems the main difficulty with in situ treatment approaches is not the actual treatment, but the implementation of the treatment and the engineering aspects. The ability to adequately deliver the treatment chemicals to the sediments appears to be the major engineering challenge in all in situ treatments conducted to date. For biological/chemical treatment, chemicals or microorganisms can be delivered with an injection bar as was demonstrated in Hamilton Harbor, or by incorporating the materials into a solid media, as evaluated in the microencapsulation study and proposed for the biological carpet treatment. Because mixing has been identified as a principal factor for solidification, in situ solidification may be even more challenging than other in situ treatment technologies.
Despite the disadvantages with in situ treatment, there continues to be some interest in using this technology. As mentioned before, evidence suggests that removal techniques (i.e., dredging) often leave the top layer of sediment more contaminated than before it was dredged. Also, dredging often does not remove all of the contaminated sediment, as was seen in Hamilton Harbor. Despite continued work to improve the environmental impacts of dredging, in situ treatment can still offer some distinct advantages. Besides reducing the possible resuspension and the volatilization of contaminants associated with dredging, in situ treatment is the only remediation technique that destroys or treats the contaminants in place. Finally, it is estimated that in situ treatment will cost approximately 20% of the cost of dredging and disposal in a confined disposal facility (Murphy et al, 1995b). While in situ treatment is likely to be more expensive than in situ capping, it appears to be less costly than any remediation technique involving removal of the sediments.
Very few in situ treatment field projects have been completed to date, and there is a need for further research to be conducted to improve this innovative technology. However, despite limited success, in situ treatment is still a viable option and should be the first option considered in any contaminated sediments remediation (Hayes, 1998).
Abramowicz, David A., Harkness, Mark R., McDermott, John B., and Salvo, Joseph J. 1992. 1991 In Situ Hudson River Research Study: A Field Study on Biodegradation of PCBs in Hudson River Sediments, Final Report. Prepared by General Electric Company Corporate Research and Development, February.
Babin, Jay. 1998. Personal communication. Limnofix Inc./Golder Associates, Mississauga, Ontario, Canada.
Bishop, Dollop F. 1996. Bioremediation of Sediments. From the Seminar Series on Bioremediation of Hazardous
Waste Sites: Practical Approaches to Implementation. U.S. EPA National Risk Management Research Laboratory, Cincinnati, OH.
Bishop, Dollop F. 1998. Personal communication. U.S. EPA National Risk Management Research Laboratory,
Cincinnati, OH.
Bokuniewicz, Henry J., et al. 1997. Contaminated Sediments in Ports and Waterways - Cleanup Strategies and Technologies. National Research Council prepared by Committee on Contaminated Marine Sediments, National Academy Press, Washington, D.C.
Chowdhury, Ajit K., Stolzenburg, Thomas R., Stanforth, Robert R., Warner, Michael A., and LaRowe, Mark, E. 1996. Underwater Treatment of Lead-Contaminated Sediment. Remediation: The Journal of Environmental Cleanup Costs, Technologies and Techniques, Vol. 6, No. 2, Spring.
Cowgill, David. 1998. Personal communication. U.S. EPA Region 5, Chicago, IL.
Development Programme Treatment Processes, Phase 1, 1991. Summary, conclusions and recommendations, presented to Dutch Parliament.
Fitzpatrick, William. 1998. Personal communication. Wisconsin Department of Natural Resources, Madison, WI.
Gatchett, Annette. 1998. Personal communication. Director of U.S. EPA’s Superfund Innovative Technology Evaluation Program, Cincinnati, OH.
Haggard, John. 1998. Personal communication. General Electric Corporate Environmental Programs, Albany, NY.
Hayes, Donald. 1998. Personal communication. Professor at the University of Utah, Salt Lake City, UT.
Heitkamp, Michael A., and Stewart, William P. 1996. A Novel Porous Nylon Biocarrier for Immobilized Bacteria. Applied and Environmental Microbiology, Vol. 62, No. 12, pp. 4659-4662, December.
Irvine, K.N., Droppo, I.G., Murphy T.P., and Stirrup, D.M. 1997. Annual Loading Estimates of Selected Metals and PAHs in CSOs, Hamilton, Ontario, using a Continuous PCSWMM Approach, National Water Research Institute, Burlington, Ontario.
Kita, D., and Kubo, H. 1983. Several solidified sediment examples. Proceedings of the 7th U.S./Japan Experts Meeting: Management of Bottom Sediments Containing Toxic Substances, 2-4 November 1981, New York City, U.S.A. U.S. Army Corps of Engineers, Water Resource Support Center (ed.), pp. 192-210.
Lee, Paul. 1997. PCB Contamination in the Chesapeake Bay Area: A Bioremediation Proposal. http://bordeaux.uwaterloo.ca/biol447new/97assignment2/PLEE_PBC.html.
Letterman, Raymond D., and Meng, X.M. 1990. Immobilization of Cadmium in Sediment by Treatment with Aluminum Hydrolysis Products. Environmental Engineering, edited by Charles, R., and O’Melia, P.E. Proceedings of the 1990 Specialty Conference.
Lin, Jian-Er, Mueller, James G., and Pritchard, Parmely H. 1992. Use of Encapsulated Microorganisms as Inoculants for Bioremediation. Presented at the I&EC Special Symposium, American Chemical Society, Atlanta, GA, September 21-23.
Meyers, Thomas E., Averett, Daniel E., Fleming, Elizabeth C., and Channel, Michael G. 1994. Solidification/stabilization technology for reducing the mobility of heavy metals in polluted sediments. Proceedings of 15th U.S./Japan Experts Meeting: Management of Bottom Sediments Containing Toxic Substances, 19-21 November 1991, San Pedro, CA. T.R. Patin (ed.), U.S. Army Corps of Engineers, Water Resources Support Center, pp. 273-281.
Murphy, T.P., Moller, A., Pandey, R., Brouwer, H., Fox, M., Babin, J., and Gray, K. 1995a. St. Mary’s River - Chemical Treatment of contaminated sediments by iron injection. The Lake Huron Ecosystem: Ecology, Fisheries and Management, pp. 397-412. Edited by Munawar, M., Edsall, T., and Leach, J. SBP Academic Publishing, Amsterdam, The Netherlands.
Murphy T., Moller A., and Brouwer H. 1995b. In situ treatment of Hamilton Harbor sediment, Journal of Aquatic Ecosystem Health, Vol 4, pp. 195-203.
Murphy, Tom. 1998. Personal communication. National Water Research Institute, Burlington, Ontario, Canada.
OCETA Environmental Technology Profile. 1995. Limnofix In Situ Sediment Treatment. Http://www.oceta.on.ca/profiles/limnofix/list.html.
Pritchard, Hap. 1998. Personal communication. Section Head of Environmental Quality Sciences, U.S. Naval Research Laboratory (Code 6115), Washington, D.C.
Schulz, R.S. 1989. Mercury Fixation in Contaminated Sediments as a Management Option at Albany, Western Australia. Water Science Technology, Vol. 21, No. 2, pp. 45-51.
Shimoda, Masao. 1994. Fixation Mechanism of Toxic Heavy Metals with Cements. Proceedings of 15th U.S./Japan Experts Meeting: Management of Bottom Sediments Containing Toxic Substances, 19-21 November 1991, San Pedro, CA. T.R. Patin (ed.), U.S. Army Corps of Engineers, Water Resource Support Center, 12 pages.
Strand, Stuart. 1998. Personal communication. University of Washington, Seattle, WA.
Tabak, Henry H, and Bishop, Dolloff F. 1998. Strategies for In-Situ Biorestoration of Contaminated Sediments and Determination of Natural Recovery Rates. U.S. EPA’s National Risk Management Research Laboratory, Cincinnati, OH.
Timberlake, Dennis. 1998. Personal communication. U.S. EPA National Risk Management Research Laboratory,
Cincinnati, OH.
U.S. Environmental Protection Agency. 1990. “Contaminated Sediments: Relevant Statutes and EPA Program Activities.” EPA 506/6-90/003. Office of Water, Washington, D.C.
U.S. Environmental Protection Agency. 1993a. “Selecting Remediation Techniques For Contaminated Sediment.” EPA 823- B93-001. Office of Water, Washington, D.C.
U.S. Environmental Protection Agency. 1993b. “Questions and Answers About Contaminated Sediments.” EPA 823-F-93- 009. Office of Water, Washington, D.C.
U.S. Environmental Protection Agency. 1994. “ARCS Remediation Guidance Document.” EPA 905-R94-003. Great Lakes National Program Office, Chicago, IL.
U.S. Environmental Protection Agency. 1997. “The Incidence and Severity of Sediment Contamination in Surface Waters of the United States - Volume 1: National Sediment Quality Survey.” EPA 823-R-97-006. Office of Water, Washington, D.C.
U.S. Environmental Protection Agency. 1998. “Fact Sheet Contaminated Sediment: EPA’s Report to Congress.” EPA 823-F- 98-001. Office of Water, Washington, D.C.
Wada, N. 1992. Solidification techniques for dredged bottom sediments. Proceedings of the 14th U.S./Japan Experts Meeting: Management of Bottom Sediments Containing Toxic Substances, 27 February - 1 March 1990, Yokohama, Japan. T.R. Patin (ed.), U.S. Army Corps of Engineers, Water Resource Support Center, pp. 223-237.
Warner, Michael A. 1998. Personal communication. Civil Engineer at RMT Inc., Madison, WI.
White, Erin. 1998. Realizing Remediation: A Summary of Contaminated Sediment Remediation Activities in the Great Lakes Basin. Project Officer, Bolattino, Callie, U.S. EPA’s Great Lakes National Program Office, Chicago, IL.
APPENDIX - LIST OF CONTACTS FOR MAJOR IN SITU TREATMENTS
| Project Location |
Contact Name |
Organization |
Contact Information |
|---|---|---|---|
| Hamilton Harbor and St. Mary’s River, Canada, as well as Salem, MA (Future Project) |
Jay Babin |
Limnofix Inc. |
Golder Associates 2180 Meadowvale Blvd. Mississauga, Ontario Canada L5N5S3 Phone: (905) 567-6100 (Ext. 748) E-mail: JBabin@golder.com |
| Hamilton Harbor and St. Mary’s River, Canada |
Tom Murphy |
National Water Research Institute, Environment Canada |
National Water Research Institute 867 Lakeshore Road, P.O. Box 5050 Burlington, Ontario Canada L7R4A6 Phone: (905) 336-4602 Fax: (905) 336-8901 E-mail: Tom.Murphy@CCIW.ca |
| Fox River, WI |
Michael Warner |
RMT Inc. |
RMT, Inc. 744 Heartland Trail Madison, WI 53717 Phone: (608) 831-4444 E-mail: mick.warner@rmtinc.com |
| Manitowoc River, WI |
Bill Fitzpatrick |
State of Wisconsin Department of Natural Resources |
Bureau of Watershed Management 101 S. Webster St., Box 7291 Madison, WI 53707-7921 Phone: (608) 266-9267 Fax: (608) 267-2800 E-mail:FITZPW@DNR.STATE.WI.US |
| Hudson River, NY |
John Haggard |
General Electric |
General Electric 1 Computer Drive South Corporate Environmental Programs Albany, NY 12205 Phone: (518) 458-6619 Fax: (518) 458-1014 E-mail: John.Haggard@corporate.ge.com |