Per- and Polyfluoroalkyl Substances (PFAS)
Chemistry and Behavior
- Overview
- Policy and Guidance
- Chemistry and Behavior
- Occurrence
- Toxicology
- Site Characterization and Analytical Methods
- Remediation Technologies
- Conferences and Seminars
- Additional Resources
Perfluorooctane Sulfonic Acid (PFOS)
The structure of perfluorooctane sulfonic acid (C8F17SO3H, CAS Number 1763-23-1) is shown in Figure 1. PFOS has an 8-carbon fully fluorinated backbone attached to a sulfonic acid functional group (Buck et al. 2011). PFOS is a strong acid that is generally present in solution as the perfluorooctane sulfonate anion (C8F17SO3-, CAS Number 45298-90-6) (USEPA 2016). Although the PFOS anion has the ability to sorb to soils, sediments, or sludge, it is expected to be mobile in the aqueous phase at equilibrium (3M Company 2003).
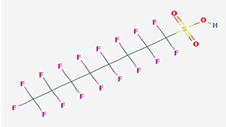
Source: PUBCHEM
PFOS is stable in environmental media because it is resistant to environmental degradation processes, such as biodegradation, photolysis, and hydrolysis. In water, no natural degradation has been demonstrated, and dissipation is by advection, dispersion, and sorption to particulate matter. PFOS has low volatility in ionized form, but can adsorb to particles and be deposited on surface soil and into water bodies (USEPA 2016).
The availability of naturally occurring cations in water affects the solubility of PFOS and its salts, as discussed in Brooke et al. 2004 and OECD 2002. For example, the solubility of the PFOS potassium salt decreases significantly with increasing salt content of the water (OECD 2002).
Table 1. Chemical and Physical Properties of PFOS |
||
|---|---|---|
| Property | PFOS, acidic forma | Source |
| Chemical Abstracts Service Registry No. (CASRN)b | 1763-23-1 | |
| Chemical Abstracts Index Name | 1,1,2,2,3,3,4,4,5,5,6,6,7,7,8,8,8-heptadecafluoro- 1-octanesulfonic acid | |
| Synonyms | Perfluorooctane sulfonic acid; heptadecafluoro- 1-octane sulfonic acid; PFOS acid | |
| Chemical Formula | C8HF17O3S | |
| Molecular Weight (g/mol) | 500.13 | HSDB (2012); Lewis (2004); SRC (2016) |
| Color/Physical State | White powder (potassium salt) | OECD (2002) |
| Boiling Point | 258-260 degrees Celsius (°C) | SRC (2016) |
| Melting Point | No data | |
| Vapor Pressure | 2.0X10-3 mm Hg at 25 °C (estimate) | HSDB (2012) |
| Henry's Law Constant | Not measurable | ATSDR (2015) |
| Kow | Not measurable | ATSDR (2015); EFSA (2008) |
| Koc | 2.57 | Higgins and Luthy (2006) |
| Solubility in Water | 680 mg/L | OECD (2002) |
| Half-life in Water | Stable | UNEP (2006) |
| Half-life in Air | Stable | UNEP (2006) |
|
Notes: Kow = octanol-water partition co-efficient; Koc = organic carbon-water partitioning coefficient a PFOS is commonly produced as a potassium salt (CASRN 2795-39-3). Properties specific to the salt are not included. b The CASRN given is for linear PFOS, but the toxicity studies are based on a mixture of linear and branched; thus, the RfD applies to the total linear and branched. Source: USEPA 2016
|
||
A report by Environment Canada (2006) discusses and provides a list of PFOS and its precursors.
References
3M Company. 2003. Environmental and Health Assessment of Perfluorooctane Sulfonic Acid and Its Salts.![]() 152 pp.
152 pp.
Brooke, D., A. Footitt, and T.A. Nwaogu. 2004. Environmental Risk Evaluation Report: Perfluorooctanesulphonate (PFOS).![]() U.K. Environment Agency. 104 pp.
U.K. Environment Agency. 104 pp.
Buck, R.C. et al. 2011. Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integrated Environmental Assessment & Management 7(4):513-541.
Environment Canada. 2006. Ecological Screening Assessment Report on Perfluorooctane Sulfonate, Its Salts and Its Precursors that Contain the C8F17SO2 or C8F17SO3, or C8F17SO2N Moiety.![]() 81 pp.
81 pp.
OECD (Organisation for Economic Co-operation and Development). 2002. Hazard Assessment of Perfluorooctane Sulfonate (PFOS) and Its Salts.![]() ENV/JM/RD(2002)17/FINAL, 362 pp.
ENV/JM/RD(2002)17/FINAL, 362 pp.
USEPA (U.S. Environmental Protection Agency). 2016. Drinking Water Health Advisory for Perfluorooctane Sulfonate (PFOS).![]() Office of Water, EPA 822-R-16-004, 88 pp.
Office of Water, EPA 822-R-16-004, 88 pp.
![]() Drinking Water Health Advisory for Perfluorooctane Sulfonate (PFOS)
Drinking Water Health Advisory for Perfluorooctane Sulfonate (PFOS)
U.S. EPA, Office of Water.
EPA 822-R-16-004, 88 pp, 2016
Chapter 2, Nature of the Stressor, contains information relevant to the chemistry and behavior of PFOS.
![]() Ecological Screening Assessment Report on Perfluorooctane Sulfonate, Its Salts and Its Precursors that Contain the C8F17SO2 or C8F17SO3, or C8F17SO2N Moiety
Ecological Screening Assessment Report on Perfluorooctane Sulfonate, Its Salts and Its Precursors that Contain the C8F17SO2 or C8F17SO3, or C8F17SO2N Moiety
Environment Canada, 81 pp, 2006
This report contains chapters on identity, uses, and sources of release; environmental fate, exposure, and effects; environmental concentrations; key toxicological studies; and risk quotient analyses. The appendices include a list of PFOS and its precursors as well as PFOS concentrations in selected wildlife in North America and circumpolar regions.
![]() Environmental Risk Evaluation Report: Perfluorooctanesulphonate (PFOS)
Environmental Risk Evaluation Report: Perfluorooctanesulphonate (PFOS)
Brooke, D., A. Footitt, and T.A. Nwaogu.
Environment Agency, 104 pp, 2004
With a focus on the PFOS anion, sections that expand upon PFOS chemistry and behavior include Purity/Impurities, Additives; Physico-Chemical Properties; Uses; and Environmental Fate and Distribution.
Guidance for the Inventory of Perfluorooctane Sulfonic Acid (PFOS) and Related Chemicals Listed under the Stockholm Convention on Persistent Organic Pollutants, Revised Draft
United Nations Environment Programme, UNEP/POPS/COP.7/INF/26, 125 pp, revised Jan 2017
To provide background for developing a chemical inventory, this document discusses commercial production and use of PFOS and related compounds by specific industries, identifying products, stockpiles, and wastes generated.
Guidance on Best Available Techniques and Best Environmental Practices for the Use of Perfluorooctane Sulfonic Acid (PFOS) and Related Chemicals Listed under the Stockholm Convention on Persistent Organic Pollutants
United Nations Environment Programme, UNEP/POPS/COP.7/INF/21, 73 pp, 2017
This document has chapters on process descriptions of current and alternative PFOS chemistry and processes; best available techniques/best environmental practices (BAT/BEP) principles for chemical management of PFOS; and specific BAT/BEP measures by process category.
![]() Hazard Assessment of Perfluorooctane Sulfonate (PFOS) and Its Salts
Hazard Assessment of Perfluorooctane Sulfonate (PFOS) and Its Salts
Organisation for Economic Co-operation and Development (OECD), Environment Directorate, Joint Meeting of the Chemicals Committee and the Working Party on Chemicals, Pesticides and Biotechnology, ENV/JM/RD(2002)17/FINAL, 362 pp, 2002
An overview of PFOS and salts includes physicochemical properties, production and use of PFOS, and environmental exposure and fate. Also provided is an extensive discussion of human health hazards and potential ecological hazards. A large appendix contains robust summaries of key ecotoxicology studies.





