- Recent Developments in Analytical Methods for Emerging Contaminants
- Fluidized Bed Reactor and Ion Exchange Systems Added for Perchlorate Removal
- Ultraviolet and Hydrogen Peroxide Treatment Removes 1,4-Dioxane from Multiple Aquifers
- Containerized Wetland Bioreactor Evaluated for Perchlorate and Nitrate Degradation
Recent discoveries of previously undetected but potentially hazardous chemical compounds in environmental media have focused attention on the need for better understanding of these "emerging contaminants." Increased identification of emergent contaminants in soil, sediment, and ground water is due in great part to the development of new analytical methods, as well as increased field testing. The high persistence and ground-water mobility of many emergent contaminants often require the use of ex-situ cleanup technologies. This issue of Technology News and Trends highlights new analytical methods and innovative cleanup technologies for two emergent contaminants, perchlorate and 1,4-dioxane.
Recent Developments in Analytical Methods for Emerging Contaminants
CLU-IN Resources
The CLU-IN Contaminant Focus area (http://www.cluin.org/
contaminantfocus/) bundles information associated with cleanup of individual contaminants and contaminant groups, which currently include
1,4-dioxane, chromium VI, MTBE, perchlorate, PCBs, and trichloroethene. Information is presented on policy and guidance, chemistry and behavior, environmental occurrence, toxicology, detection and site characterization, treatment technologies, and conferences/seminars.
Development trends in analytical methods for emerging contaminants were reviewed last year by the U.S. EPA’s National Exposure Research Laboratory (NERL). In addition to methods for more widely recognized contaminants such as perchlorate and methyl tert-butyl ether (MTBE), tools were examined for new high-priority compounds, such as perfluorooctanoic acid (PFOA), polybrominated diphenyl ethers (PBDEs), pharmaceuticals, and endocrine disrupting compounds (EDCs).
Mass spectrometry (MS) plays an increasing role in contaminant identification and measurement and has been enhanced through lower detection limits, improved analytical instrumentation, and new derivization procedures. The use of low-pressure gas chromatography (GC) with GC/MS, for example, proved to be a simple but important improvement to traditional GC/MS analysis that allows for much shorter analysis times. Other analytical techniques that are used increasingly are:
- time-of-flight, quadrupole-ion trap, and Fourier transform MS;
- chiral separations, usually with chiral GC or liquid chromatography (LC) columns or with capillary electrophoresis;
- on-line coupling of extraction with separation/detection such as solid-phase extraction (SPE) or solid-phase microextraction (SPME) coupled with LC/MS;
- LC/MS/MS and GC/MS/MS;
- coupling of LC and ion chromatography (IC) with inductively coupled plasma (ICP)-MS for inorganics; and
- matrix-assisted laser desorption ionization (MALDI)-MS and electrospray ionization (ESI)-MS for microorganism/pathogen identification.
Some of the more significant research results in emerging contaminants identified during the NERL review included:
- Sensitive analytical techniques now allow for more routine detection of perchlorate. IC with ESI-MS is emerging as an important tool for analyzing perchlorate and other ionic contaminants in water due to its ability to provide high specificity/sensitivity and sub-ppb detection limits in different environmental matrices. Perchlorate in both water and soil can be detected to levels of 0.05 µg/L and 0.5 µg/kg, respectively, using new LC/ESI-MS/MS methods.
- Early research on the fluorochemicals, PFOA and perfluorooctane sulfonate (PFOS), reveals that both compounds display unexpected toxicity, persistence, and bioaccumulative ability. LC/ESI-MS/MS commonly is used to obtain ppb- to ppt-level measurements in environmental and biological samples. Little information is available, however, on fluorochemical exposure pathways, environmental occurrence, environmental fate, or optimal cleanup technologies.
- PBDEs, which are commonly used as flame retardants, also emerged due to their environmental persistence and potential adverse developmental effects. While PBDEs are not regulated in the U.S., Europe has established a directive for control of their emissions. GC with EI-MS and negative ion chemical ionization (CI) currently are used to detect PBDEs in biological samples at low pg/g levels.
- Newly-discovered EDCs, for which EPA is required to develop screening and testing strategies, were the subject of increased studies. Researchers are testing new GC/MS and LC/MS/MS methods, as well as integrated methods employing both biological and chemical procedures, for measuring EDCs in environmental samples.
- Increased discovery of pharmaceuticals in surface water and ground water has led to intensive study of their potential estrogenic effects on humans and wildlife. Technological improvements now allow field staff to measure the occurrence of pharmaceuticals in surface and ground water and to examine their fate in wastewater, where a few have been found to be resistant to treatment. The high polarity of pharmaceuticals requires the use of LC/MS and LC/MS/MS or an efficient derivatization procedure combined with GC/MS and GC/MS/MS for analysis at low ng/L levels in environmental samples.
- Improved analytical tools were developed for herbicide and pesticide degradation products, which can exist in the environment at greater concentrations and higher frequencies than their parent compounds. The development of chiral chromatography now provides for analysis of individual isomers indicating the occurrence, degradation, and environmental fate of chiral pesticides. A new SPE-LC/ESI-MS method also was developed to measure acetanilide herbicide (e.g., alachlor) degradation products in ground water. For soil, an ion trap-LC/MS/MS method was developed to identify additional metabolites of trifluralin, a complex pesticide. Analytical instrumentation advanced significantly through development of a supersonic GC/MS technique providing enhanced molecular ions and rapid measurement of pesticides in complex matrices.
- Recent discoveries of emerging disinfection byproducts (DBPs) such as nitrosodi- methylamine (NDMA), halonitromethanes, and iodinated DBPs (including iodo-acids) were made in drinking water. NDMA also has been found as a contaminant in ground water. Analytical techniques for these emerging DBPs involve derivatization with GC-ECD, SPE-GC/MS, and purge-and-trap-GC/MS. A highly sensitive method for NDMA involves the use of GC/CI-MS/MS, which allows low ng/L (ppt) detection. Exploration of LC/MS methods continues in efforts to identify highly polar DBPs potentially missed in traditional GC/MS methods.
- National security issues have generated advancements in methods for rapid detection of chemical and biological warfare agents. New developments include an automated SPME-GC/MS method for measuring 2-chlorovinylarsonous acid in humans; a packed capillary LC/ESI-MS method for identifying chemical warfare agents, their degradation products, and related compounds in soil samples; and MALDI-MS and LC/ESI-MS methods for identifying botulinum toxins.
As part of this review, NERL identified websites containing related information on emergent contaminants (Figure 1). Complete results of the review are available in the January 15, 2004, issue of Analytical Chemistry (Richardson, S. D., Anal. Chem. 2004, 76 (12), 3337-3364).
|
U.S. EPA Websites www.epa.gov |
Topic Agency-wide site, with searchable link |
Additional Website |
CA Department of Health Services, NDMA |
Contributed by Susan D. Richardson, Ph.D., EPA/NERL (706-355-8304 or richardson.susan@epa.gov)
This article has been reviewed in accordance with the U.S. EPA’s peer and administrative review policies and approved for publication.
Fluidized Bed Reactor and Ion Exchange Systems Added for Perchlorate Removal
Ground water beneath the GenCorp Aerojet facility in Rancho Cordova, CA, is actively treated by multiple-technology, ex-situ treatment systems, three of which are designed to remove commingled perchlorate and trichloroethene (TCE). Perchlorate removal is achieved through use of either a fluidized bed reactor (FBR) or an ion exchange unit. The first perchlorate-specific technology to be implemented was the FBR, which was added to an existing ground-water extraction and treatment system in 1998 shortly after perchlorate was discovered in the western portion of the site. In 2002, the ion exchange unit was added to a separate treatment system to address perchlorate in another TCE plume located in the northern portion of the site. Both perchlorate treatment components consistently have achieved perchlorate concentrations below the 4 µg/L detection limit in post-treatment effluent.
An FBR is a columnar reactor that optimizes biological treatment of ground water through use of activated carbon or sand serving as a medium for biological growth. Water flows upward through the reactor at a sufficient velocity to expand and fluidize the bed. The design allows for a large inventory of biomass to be maintained within the reactor while maximizing contact between microorganisms and contaminants. Additional components of the system include continuous sand filters to remove solids (primarily waste biomass) and equipment to handle solids.
At Rancho Cordova, ground water extracted through 21 wells is diverted to a system employing four 14-foot-diameter, 22-foot-high bioreactors operating in parallel (Figure 2). Each reactor contains 44,000 pounds of carbon substrate to which ethanol is added as an electron donor. The FBRs are equipped with a bed-cleaning eductor system that typically adjusts the reactor bed height once each day. Retention time within each reactor averages 12 minutes. The system has shown capability to treat up to 5,400 gpm and currently operates at a rate of approximately 5,000 gpm.

Following FBR treatment, ground water is treated in a UV/oxidation system to destroy NDMA and an air stripping system to remove VOCs. Final treated effluent is released onsite to surface water. Approximately 90,000 gallons of wastewater (0.5% solids) generated in the FBR process each week are discharged to the sanitary sewer under an existing permit. The FBR process completely destroys perchlorate, without the need for offsite waste disposal. To date, the FBR system has removed 60 tons of perchlorate mass while treating 8.7 billion gallons of ground water. Operation and maintenance of the system is estimated at $0.15/1,000 gallons of treated water.
Supplied by 22 ground-water extraction wells, the ion exchange unit operates in a separate area of the facility. Prior to ion exchange treatment, ground water is exposed to a dual/parallel air stripping system for VOC removal, adjusted for pH by adding carbon dioxide, and filtered through 5-micron bags for particulate removal.
Ground water then is routed to twelve 48-inch-diameter ion exchange vessels arranged in two parallel banks of six (Figure 3), which allows for operation of a two-stage "lead/lag" treatment process operating at a rate of 980 gpm. Each vessel contains 60 ft3 of a "once-through" gel anion resin targeting perchlorate removal. The lead stage removes the bulk of perchlorate from ground water in the first bank of vessels. Lag-stage operations in the second bank continuously polish lead-stage effluent to achieve a perchlorate concentration below 4 µg/L.

Final effluent from the ion exchange system returns to the subsurface through six onsite recharge wells. Once the lead-stage vessels are exhausted, reaching their pre-set level of perchlorate breakthrough (30%), they are removed from the system. The lead vessels then are replaced with ones containing fresh resin and switched to a lag-stage position through valve changes. Spent resin is removed from the vessels and disposed at an approved landfill as non-hazardous waste.
Design of the ion exchange system allows for future addition of ion exchange modules to accommodate higher process flow if more recovery wells are required. Use of a lead/lag design optimizes expenditure of the ion exchange resin by allowing higher perchlorate breakthrough concentrations in effluent from the lead vessel, while still reaching target concentrations of perchlorate in the lag-stage effluent. On average, resin replenishment in each vessel is required every 45 days. A total of 2,500 ft3 of resin was expended at an estimated cost of $625,000 (including labor and disposal) over the two years of operation. Use of the ion exchange system has resulted in removal of 1,480 pounds of perchlorate mass while treating 850 million gallons.
Aerojet estimates that operation and maintenance costs for the combined treatment system average $0.24/1,000 gallons. The FBR and ion exchange systems are anticipated to operate indefinitely. As an active member of the national Perchlorate Study Group, Aerojet continues to evaluate additional technologies that may accelerate ground-water cleanup. Preliminary data from current pilot tests on the use of in-situ bioremediation to remove perchlorate in soil and ground water show promising results.
Contributed by Chris Fennessy, GenCorp Aerojet (916-355-3341 or christopher.fennessy@aerojet.com), Alex MacDonald, California Regional Water Quality Control Board (916-464-4625 or amacdonald@waterboards.ca.gov), Bill Guarini, Shaw Environmental (609-895-5384 or william.guarini@shawgrp.com), and Tim Peschman, USFilter (651-638-1325 or peschmant@usfilter.com)
Ultraviolet and Hydrogen Peroxide Treatment Removes 1,4-Dioxane from Multiple Aquifers
Pall Life Sciences (PLS) operates a full-scale UV/hydrogen peroxide (H202) system at its facility near Ann Arbor, MI, to remediate ground water containing high concentrations of 1,4-dioxane. Over 60,000 pounds of 1,4-dioxane have been extracted from the ground water using this combined technology since treatment began in 1997. The Michigan Department of Environmental Quality (DEQ) is overseeing cleanup of the PLS site.
As former owners of the property, Gelman Sciences Inc. used large quantities of 1,4-dioxane from 1966-1986 for the production of microporous filters. Onsite wastewater disposal resulted in the release of 1,4-dioxane to ground water, where multiple plumes developed. When 1,4-dioxane contamination was identified in the mid-1980s, ground-water concentrations were as high as 221,000 µg/L, and several local drinking water wells were affected. As defined by the State of Michigan residential drinking water criterion of 85 µg/L, the plumes collectively encompass an area of approximately 0.6 mi2.
Ground water is generally shallow, averaging 15 feet below ground surface. The subsurface consists of glacial deposits that are up to 300-feet thick and overlie the Mississippian-aged Coldwater Formation (primarily shale), which serves as the lower boundary of contamination. At least two primary sand/gravel aquifers with differing ground-water flow directions and rates exist within the area’s clay-rich deposits. 1,4-Dioxane has migrated through these aquifers at least 8,000 feet from the source areas.
Figure 4 portrays the general scheme of the PLS treatment system. Eighteen purge wells, including a 4,479-ft long horizontal well, are used to extract and divert ground water to the PLS site. In a lined pretreatment pond known as "the Red Pond," purged water is mixed with sulfuric acid (93% solution by volume) in order to lower the pH to 3.8, which earlier studies showed optimal for UV/H202 chemical reactions.
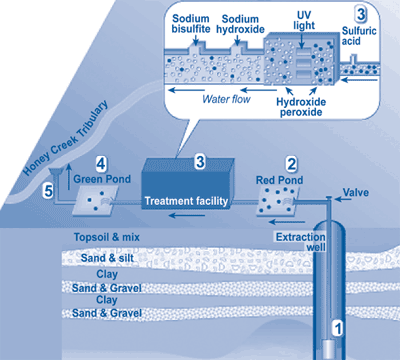
In the first step of treatment, a 50% H202 solution is injected into the treatment line and mixed with ground water by a static mixer. Water then passes through a multiple-chamber UV system consisting of 22 lamps, where it is exposed to UV radiation for approximately 5 seconds. After UV/H202 treatment, the pH of the treated water is raised to approximately 6.9 by adding sodium hydroxide (40% by volume) in order to meet surface water discharge requirements of 6.5-9.0. Sodium bisulfate also is added to remove excess H202 prior to discharge onsite to a holding pond known as "the Green Pond." The discharge is monitored daily to ensure compliance with state requirements. Under its NPDES permit, PLS can extract, treat, and discharge up to 1,300 gpm. The discharge is monitored daily to ensure compliance with State requirements prior to its release into an unnamed tributary of Honey Creek, which flows into the Huron River.
Ground water is monitored routinely at 50-100 locations. Although treatment has only slightly reduced the plumes' areal extent, 1,4-dioxane concentrations within the plumes have decreased significantly due to mass removal. A nearly 100-fold reduction in 1,4-dioxane concentrations has been observed in some areas. Maximum 1,4-dioxane concentrations in the plume are now less than 10,000 µg/L.
Large volumes of reactive chemicals are required for treating ground water using the current UV/H202 treatment system. The electrical demand also is high, averaging approximately 660 kW/hr/day at a cost of approximately $850/day. The overall treatment cost using the UV/H202 treatment system is approximately $3.50 /1,000 gallons. PLS plans to convert the UV/H202 system to an ozone/H202-based technology in 2005 in order to reduce H202 consumption by 50% and eliminate the need for sulfuric acid and sodium hydroxide.
In the new system, which PLS is in the final stage of designing, water from the extraction wells will be transferred to a pre-treatment pond to settle non-soluble iron. Following settlement, water will be transferred to a central environmental building for introduction of H202 through injector quills. The water then will be transferred to separate units where ozone will be administered through a series of venturi injectors. The treated water will be transferred back to the environmental building for addition of post-treatment chemicals, if necessary, and allowed to settle in a second pond prior to surface water discharge. The new system will reduce 1,4-dioxane concentrations at a rate similar to UV/H202 treatment while minimizing production of bromate (a common byproduct of ozone treatment of ground water containing bromide). Treatment costs using the ozone/H202-based technology are anticipated to be approximately $1.50/1,000 gallons.
Evaluation of alternative cleanup remedies capable of removing contaminant mass from the aquifers while maintaining hydraulic conditions is underway. Last year, PLS conducted an in-situ chemical oxidation (ISCO) pilot test involving injection of H202 and Fenton’s reagent (iron catalyst) into one of the confined aquifers, but a minimal reduction of 1,4-dioxane concentrations was achieved. Additional field testing of ISCO using ozone resulted in a slightly higher rate of removal, but bromate formation exceeded the 10 µg/L maximum contaminant level. Use of the current pump-and-treat method is anticipated to continue until the 1,4-dioxane target cleanup criteria are reached.
Contributed by Jim Brode, Fishbeck Thompson Carr and Huber (269-375-3824 or jwbrode@FTCH.com), Farsad Fotouhi, Pall Corporation (734-913-6130 or farsad_fotouhi@pall.com), and Sybil Kolon , Michigan DEQ (517-780-7937 or kolons@michigan.gov)
Containerized Wetland Bioreactor Evaluated for Perchlorate and Nitrate Degradation
The U.S. Department of Energy (DOE) and Lawrence Livermore National Laboratory (LLNL) designed and constructed an innovative containerized wetland (bioreactor) system that began operation in November 2000 to biologically degrade perchlorate and nitrate under relatively low-flow conditions at a remote location at Site 300 known as Building 854. Since initial start-up, the system has processed over 3,463,000 liters of ground water and removed over 38 grams of perchlorate and 148 kilograms of nitrate.
Site 300 is operated by the University of California as a high-explosives and materials testing facility supporting nuclear weapons research. The 11-square-mile site located in northern California was added to the NPL in 1990, primarily due to the presence of elevated concentrations of VOCs in ground water. At the urging of the regulatory agencies, perchlorate was looked for and detected in the ground water in 1999. VOCs, nitrate, and perchlorate were released into the soil and ground water in the Building 854 area as the result of accidental leaks during stability testing of weapon components or from waste discharge practices that are no longer permitted at Site 300.
Design of the wetland bioreactors was based on earlier studies showing that indigenous chlorate-respiring bacteria could effectively degrade perchlorate into nontoxic concentrations of chlorate, chlorite, oxygen, and chloride. Studies also showed that the addition of organic carbon would enhance microbial denitrification. Early onsite testing showed acetic acid to be a more effective carbon source than dried leaf matter, dried algae, or milk replacement starter (a nutrient and carbon source used in a Department of Defense phytoremediation demonstration).
Using solar energy, ground water is pumped into granular activated carbon canisters to remove VOCs (Figure 5). Following removal of VOCs, the effluent, which contains approximately 46 mg/L of nitrate and 13 µg/L of perchlorate, is gravity-fed continuously into two parallel series of two 1,900-L tank bioreactors. Each bioreactor contains coarse, aquarium-grade gravel and locally obtained plant species such as cattails (Typha spp.), sedges (Cyperus spp.), and indigenous denitrifing microorganisms. No inocula were added to the system. Ground water initially was allowed to circulate through the bioreactor for three weeks to acclimate the wetland plants and to build a biofilm from indigenous flora. Sodium acetate is added to the first bioreactor in each of the two series to promote growth and metabolic activity of rhizome microorganisms. The split flow from each series is combined and flows through two back-up ion exchange columns to assure complete perchlorate removal. Effluent from the ground-water treatment system is monitored and discharged to an infiltration trench in accordance with the Substantive Requirements for Waste Discharge issued by the California Regional Water Quality Control Board.
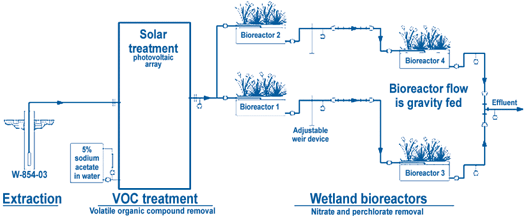
The solar-powered facility operates 10-15 hr/day, depending on cloud cover, hours of sunlight, and battery storage capacity. An active flow rate of 3.8 L/min is set to provide a minimum reactor hydraulic retention time (HRT) of 17 to 20 hours. As plants mature, the HRT requirement will increase due to accumulation of organic debris and rootlets, which decrease the available pore water space in the tank. Test data showed that degradation of perchlorate and nitrate, without an added carbon source, required HRTs of four days and 20 hours, respectively. In the presence of a 0.25 g/L solution of sodium acetate, the HRT decreased to 0.5 days.
Treatment system samples are collected quarterly from the influent and monthly from the effluent and analyzed in a laboratory for VOCs, nitrate, and perchlorate. Laboratory analysis for perchlorate in influent and effluent ground water uses ion chromatography in accordance with EPA methods 300.0 and 314.0. Analytical results indicate that the contained wetland reduces perchlorate concentrations from 14-27 µg/L to less than 4 µg/L, which is the analytical reporting limit. The State’s current public health goal is 6 µg/L. In addition, nitrate concentrations are decreased from 48 mg/L to below the 45mg/L discharge requirement. Portable field instruments are used to collect and analyze samples for pH, electrical conductivity, and temperature. In addition, treatment-system optimization samples are collected quarterly from the solar unit effluent and the bioreactor effluent for analysis of VOCs, nitrate, perchlorate, and dissolved oxygen. Over the course of operation, perchlorate has been periodically detected in the bioreactor effluent. Initial breakthroughs coincided with acetic acid injection system problems, which were corrected by replacing a venturi-type pump with a peristaltic pump. More recent breakthroughs were corrected by removing plant material from the bioreactor.
Operation of the wetland bioreactor for more than four years resulted in a stable ecosystem of indigenous microorganisms. Dominant organisms were identified through use of gradient gel electrophoresis conducted on sediment samples taken from noncontinuous, vertical coring of the bioreactor. The bacteria species identified from reactor gravel closely affiliated with species commonly distributed in soils, mud layers, and fresh water. Most of the bacteria (Pseudomonas, Acinetobacter, Halomonas, and Nitrospira) respire aerobically or anaerobically with nitrate as the terminal electron acceptor. Several identified genera (Rhizobium, Acinetobacter, and Xanthomonas) are capable of fixing atmospheric nitrogen into a combined form (ammonia) utilizable by host plants. Isolates from the Proteobacteria class, known for its ability to reduce perchlorate, also were identified.
Environmental conditions in the wetland bioreactor fluctuated with seasonal changes, even in California’s temperate climate. Seasonal average ambient air temperature ranged between 7 to 11°C during the cold season, and between 17 and 26°C during the warm season. Depending on the time of day, wetland plants moderated water temperature variations from 1 to 5°C. The influent water pH was about 7.5, and the effluent was about 7.1, well within regulatory discharge limits (6.5 to 8.5). The pH was potentially moderated during the growing season by biological carbon dioxide consumption (aquatic photosynthesis). Bioreactor redox potential ranged from –100 to –150 mV within a few weeks of operation and establishment of the microbial community and native plants. Measurements show that active bacterial growth is consuming oxygen within the bioreactor, generally causing redox values to be in an anaerobic range (<0.5 mg/L for dissolved oxygen). Dissolved oxygen in the effluent water fluctuates with seasonal effects of the plant growth cycle, metabolic activity in the bioreactor, and acetic acid injection rate.
Results demonstrate that the wetland bioreactor system can successfully remove commingled perchlorate and nitrate from ground water in a relatively short time (hours versus days) when continuously provided with a carbon source. In addition, the bioreactor degrades nitrate and perchorate to non-toxic byproducts, eliminating the need for costly waste disposal of ion-exchange resin. Above-ground containerized wetlands are easy to maintain and can be moved when cleanup is complete without impacting the natural habitat.
Contributed by Valerie Dibley and Paula Krauter, LLNL, (925-422-9777 or dibley1@llnl.gov, and 925-422-0429 or krauter2@llnl.gov)





