- Hanford Demonstrates Bioimmobilization of Hexavalent Chromium in Ground Water
- SRNL Evaluates Sustainable Remediation Strategies for Metals and Radionuclides
- Bioremediation Evaluated for Long-Term Immobilization of Uranium
- Research Shows Growing Potential of Bioremediation for Arsenic and Selenium
- EPA Releases Technical Resource for MNA of Inorganics
This issue of Technology News and Trends highlights strategies for remediating sites with inorganic contaminants and radionuclides. Enhanced research has led to increased use of bioremediation as a viable technology for removing or transforming inorganic contaminants. Due to the length of time needed to address radionuclide contamination, research also focuses on the potential for monitored natural attenuation (MNA) to complement aggressive cleanup technologies.
Hanford Demonstrates Bioimmobilization of Hexavalent Chromium in Ground Water
CLU-IN Resources
CLU-IN's Contaminant Focus area provides background information on a range of issues concerning soil or ground water with arsenic or chromium (VI) contamination. Topics include contaminant chemistry and behavior, toxicology, and regulatory guidance. Visit these topics at http://clu-in.org/contaminantfocus/.
The U.S. Department of Energy (DOE) is evaluating long-term efficacy of lactate-stimulated bioreduction to treat ground water contaminated with hexavalent chromium [Cr(VI)] at Hanford's "Site 100H" along the Columbia River in Washington. The study includes identification of critical microbial community-structure changes and stressors helping to control and predict biogeochemical processes causing Cr(VI) bioimmobilization. Polylactate in the form of Hydrogen Release Compound® (HRC) was injected into the ground water in 2004. Cr(VI) concentrations now are below the drinking water standard of 10 ppb due to transformation of Cr(VI) into insoluble Cr (III) complexes, which is largely affected by bioimmobilization stressors. Common stressors identified during the study include oxygen, nitrate, salt, and sulfate.
Chromium contamination at Site 100H likely resulted from a release of sodium dichromate once used to control corrosion at Hanford's former plutonium reactor systems, and to decontaminate shut-down reactor complexes. Ground-water analysis in 2004 at Site 100H showed a Cr(VI) concentration of approximately 100 mg/L, a level unchanged over the previous 20 years. Sand and gravel extend approximately 50 feet below ground surface (bgs) at the site. The sand and gravel are underlain by clay and silt layers that in turn overlay basalt. The water table is at 42 feet bgs.
Bench-scale studies on Site 100H ground-water and sediment samples showed that introduction of various forms of lactate stimulated an increase in bacterial population exceeding 108 cells/g. This increase generated reducing conditions leading to nearly complete Cr(VI) removal from the pore solution after only three weeks of incubation. The most viable remediation alternative involved use of a diothionite reducing permeable reactive barrier (PRB), but onsite studies suggested difficulty in long-term PRB maintenance sufficient to prevent Cr(VI) breakthrough.
Field testing in 2004 involved a single injection of 40 pounds of HRC labeled with stable isotope carbon (13C) in a well extending 50 feet bgs. A multi-screened extraction well approximately 15 feet from the injection location was pumped for 27 days to obtain water samples from the sand/gravel, clay, and silt layers.
Post-injection analysis of ground water indicated an increase in the δ13C (ratio of 13C to other carbon isotopes) of dissolved inorganic carbon from 15‰ (parts per thousand) to over 50‰, exceeding the HRC's proportion and indicating that CO2 was created as a byproduct of lactate metabolism. Depletion of competing terminal electron acceptors (oxygen, nitrate, and sulfate) occurred sequentially. Evidence of subsurface lactic acid buildup further indicated that the injection stimulated bioreduction of Cr(VI) to Cr(III) through precipitation. Naturally occurring microbial reducers of the sulfate and iron (Fe) apparently maintain the presence of hydrogen sulfide and ferrous iron [Fe(II)], subsequently maintaining Cr(VI) below 5 ppb in the injection well.
DOE's "16s rDNA microarray" method was used to detect the composition and diversity of microbes in ground-water samples (Figure 1). The method is capable of identifying the 9,900 microbial species of 16S rDNA from up to 550,000 probes in a 1.28 cm² array.
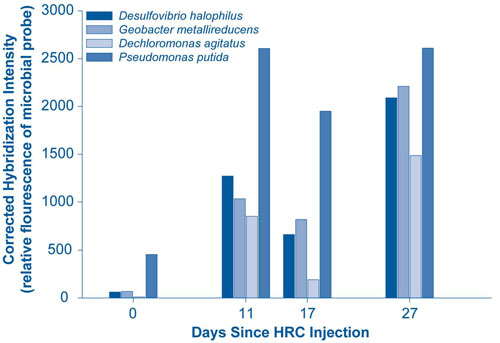
Evidence of bioreduction was supported by a decrease in reduction/oxidation from 240 to -130 mV and a decrease in oxygen content from 9 mg/L to nearly zero. Cr(VI) concentrations in the monitoring well decreased to less than 5 ppb as a result of the polylactate injection, and have remained lower than upgradient (background) concentrations. Study findings suggest that the prevailing mechanisms for Cr(VI) reduction are direct enzymatic chromate reduction and/or abiotic geochemical processes involving formation of insoluble complexes of Cr(III) with Fe(II) or sulfide (S2-). Overall study results demonstrate significant potential for using naturally occurring microorganisms to enhance in-situ Cr(VI) immobilization.
Ongoing monitoring indicates minimal chemical rebound. DOE will further examine the lateral extent and potential for rebound as well as the impact of Site 100H Cr treatment on regional ground water. Future onsite investigations also will determine the optimal number of injection wells needed for Cr bioimmobilization and the appropriate frequency of lactate reinjection. Based on the favorable Site 100 results, DOE anticipates using this remedy to control Cr(VI) concentrations in ground water at other sites such as the Idaho National Laboratory, Savannah River Site, and Pantex Plant. Updated project details are available from DOE's Office of Environmental Management at http://esd.lbl.gov/ERT/hanford100h/.
Contributed by Terry Hazen, DOE (tchazen@lbl.gov or 510-486-6223)
SRNL Evaluates Sustainable Remediation Strategies for Metals and Radionuclides
DOE's Savannah River National Laboratory (SRNL) recently initiated studies under the Department's Office of Environmental Management (EM) to identify methods for increasing sustainability of remediation addressing metal- and radionuclide-contaminated ground water. Sustainable strategies will help meet site-specific cleanup objectives, including long-term risk reduction, while minimizing maintenance, cost, and collateral environmental damage associated with remediation. Current SRNL work focuses on estimating the duration of aggressive remediation strategies before natural processes can be relied upon to return the site to pre-contamination conditions. Opportunities for using follow-on natural processes are critical to sites with contaminants known to exist in the environment for a long time.
One SRNL study area at the Savannah River Site (SRS), SC, is a 1-km2 metals/radionuclides waste site known as the "F-Area Seepage Basins," where a modified funnel-and-gate barrier system has operated since 2005 to treat ground water containing strontium (Sr)-90, uranium (U) isotopes, iodine-129, technetium-99, and tritium. The ground water is acidic (pH 3.2-4.0), a primary factor facilitating mobility of certain contaminants and associated risk drivers. In the current treatment strategy, alkaline solutions of pH 10 are injected periodically into the gates to neutralize ground water and reduce mobility of some contaminants. Injection frequency is determined by monitoring pH in wells downgradient from the injection wells; when a trigger of pH 5.5 is reached, alkaline solution is reinjected. In the three years of operation, injections were required approximately each 12 months at one gate and 18 months at the second gate.
The treatment strategy is more sustainable than the previous, inefficient, pump-and-treat (P&T) system. P&T operations cost approximately $1 million per month and produced a significant quantity of solid radioactive waste requiring disposal. The alkaline-enhanced funnel-and-gate system, however, treats all contaminants by mixing the stratified plume at the barrier wall as well as pH-sensitive contaminants such as 90Sr and uranium isotopes at the gates. Early analytical data from downgradient wells indicate the system effectively reduces concentrations of 90Sr, uranium isotopes, and tritium to below drinking water standards.
Chemical effects of the alkaline injection were demonstrated in a small-scale field test conducted over five months in 2002-2003, prior to installing the full-scale injection system. Alkaline solutions were injected below the water table at closely spaced points upgradient of the extraction well. The extraction well was pumped continually to draw injectate toward the well. Breakthrough of the injectate at the extraction well was indicated by decrease in specific conductance. The pH did not increase until four weeks after breakthrough due to the need for the injectate to first neutralize acidified surfaces of the aquifer minerals (Figure 2). Adjustments to the injection system in late December 2002 caused temporary decreases in pH and specific conductance four to five weeks later.
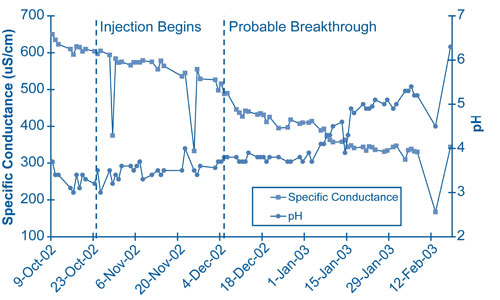
This series of chemical changes controls the time needed to return ground water to a near-natural pH of 6. When acid flux from the vadose zone becomes insignificant, uncontaminated ground water with pH near 6 migrates into the plume zone. The resulting pH front will migrate more slowly than ground water as a result of the buffering effect of acidified mineral surfaces. Duration of the injection system will depend on the migration rate of the trailing pH gradient through the treatment zone, the point at which contaminants will not remobilize. By avoiding remobilization of sequestered metals and radionculides and reducing treatment duration, sustainability of this remediation strategy is improved.
Findings from this and other waste sites suggest the rate of biogeochemical gradient migration through a treatment zone is controlled by hydrogeology and mineralogy as well as relative biogeochemical conditions of the treatment zone, plume zone, and uncontaminated zone. At sites employing treatment technologies that establish reducing zones, for example, mineralogy affects remediation sustainability. The reduced iron and manganese minerals formed during treatment will act as redox buffers and decrease migration rates of dissolved oxygen gradients, consequently remobilizing contaminants.
Understanding factors that control the rate at which F-Area Seepage Basins ground water returns to near natural conditions is a primary goal of this study, driven by SRS long-term remediation. EM's broader goal is to address these issues for other wastes and at other sites using different remediation strategies.
Contributed by Miles Denham, SRNL (miles.denham@srnl.doe.gov or 803-725-5521)
Bioremediation Evaluated for Long-Term Immobilization of Uranium
Oak Ridge National Laboratory (ORNL) and Stanford University researchers are developing a strategy for bioimmobilizing uranium in the highly contaminated subsurface of the former "S3 Ponds" site at DOE's Oak Ridge Field Research Center in Oak Ridge, TN. The approach employs a treatment train involving flow-field hydraulic control, subsurface preconditioning, and delivery of ethanol as an electron donor for in-situ U(VI) reduction/immobilization. Results of pilot-scale field tests will be integrated into DOE decision-making in 2015 regarding S3 Ponds cleanup. Additional EM assistance in evaluating site-specific environmental engineering, hydrogeology, microbiology, geo-chemistry, and physics is provided by the University of Oklahoma, Argonne National Laboratory, Michigan State University, Montana State University, and Georgia Institute of Technology.
Storage of atomic weapons production wastes in the unlined S3 Ponds from 1951 until 1984 caused extensive subsurface contaminant plumes migrating in three separate pathways, including one that discharges to a nearby creek. Remediation planning required detailed analysis of geophysical and geochemical conditions. The plume depths range from 30 to 100 ft bgs within subsurface media containing fracture densities as high as 100-200 fractures per meter. The fractures account for less than 5-10% of matrix porosity but carry more than 95% of ground-water flow. The surrounding highly porous soil and sediment have a low permeability and serve as a sink (and continuing source) of contamination.
Sample analyses indicated the highest contaminant concentrations existed in ground water at a depth of 30-50 feet bgs. To date, maximum concentrations measured for metals are 40 mg/L of depleted uranium, 540 mg/L aluminum (Al), 930 mg/L calcium (Ca), and 11-14 mg/L nickel. Perchloroethene and cis-dichloroethene in concentrations of 2-3 mg/L and 1 mg/L, respectively, are co-contaminants. Concentrations of 8,000 g/L nitrate and 1 g/L sulfate resulted from disposal of nitric and sulfuric acids that lowered the pH of the ground water to 3.4-3.6, hampering in-situ uranium bioremediation.
Solid-phase uranium in hot spots with concentrations of 200-700 mg/kg serve as a long-term source of U(VI) contamination, and aqueous-phase uranium concentrations currently exceed the federal drinking water standard by over 1,000-fold. Most of the uranium is associated with the solid phase. Laboratory and field tests showed that uranium sorption points (0.1 g soil per 15 mL solution) and desorption points (13.5 g/15 mL solution) are strongly pH dependent; high uranium adsorption was observed at pH around 6.0.
Bioremediation at the S3 Ponds relies on converting soluble U(VI) into sparingly soluble U(IV), which is more resistant to dissolution and desorption and posing less potential for ground-water migration. A range of microorganisms, including certain sulfate-reducing (SRB) and iron(III)-reducing bacteria (FeRB), can mediate this conversion. Reduced compounds such as sulfide and green rusts generated by microorganisms also can convert U(VI) to U(IV) under certain reduced conditions.
Prior to startup of the pilot-scale bioimmobilization system, a nested circulation well system containing an inner loop and an outer loop was installed to improve hydraulic control in the treatment area. Injection of clean water into the outer loop protected the inner loop from invasion of contaminated ground water. Extracted ground water was then treated through an aboveground system employing vacuum stripping to remove volatile organic compounds (VOCs), precipitation of Al and Ca sludge in a settling tank, and nitrate removal through a biological granular-activated-carbon fluidized bed reactor. Treated water was injected back into the outer loop.
In November 2002, a bromide tracer study was conducted to characterize the test area’s hydrology. The treatment-area subsurface was flushed with clean water (tap water and nitrate-free water from an aboveground treatment facility) during the following fall to achieve a pH of 4.0-4.5 and remove clogging agents and inhibitors such as Al, Ca, nitrate, and VOCs. A second clean-water flushing was performed in November 2003 to increase pH to 6.0-6.3, facilitating maximum uranium sorption capability and enhancing conditions for bioremediation. Residual nitrate in ground water was removed further by in-situ denitrification.
After an additional year of ethanol injections, uranium concentrations decreased below the maximum contaminant level (0.03 mg/L) within fast-flowing zones of the subsurface (3-8 m/day hydraulic conductivity) demonstrating that they were hydrologically connected to the injection well. Intermittent ethanol injections sequentially stimulated in-situ denitrification followed by sulfate and Fe(III) reduction and U(VI) reduction. Treatment-area sediment samples changed color from yellow-brown to dark green or black, providing further evidence of reduction and expansion within the zone of reduction. Reduction of U(VI) to U(IV) was confirmed by X-ray absorption near-edge structure spectroscopy. Before biostimulation, no U(IV) was observed in sediment samples. After biostimulation, up to 80% of the uranium sorbed to soil was reduced to U(IV).
Microbial community analysis indicated that SRB and FeRB populations are stimulated by delivery of electron donor. Prior to biostimulation, only denitrifiers were found in ground water, at an extremely low level (3 cells/mL). The amount of bacterial DNA also was too low to extract from sediment samples. Most probable estimates for denitrifiers, SRB, and FeRB in sediments (cells/g dry weight) after biostimulation increased to 107-108. Post-treatment tests indicate that microorganisms capable of reducing U(VI) to U(IV) (including SRB Desulfovibrio, Desulfoporosinus, and Desulfotomaculum spp. and FeRB Geobacter and Anaeromyxobacter spp.) were present in both ground water and sediment. These results confirm that biostimulation promoted biotic and secondary abiotic reductions of U(VI) in ground water.
The pilot system continues to operate to evaluate stability of reduced uranium. Bioreduced U(IV) was stable following suspension of ethanol delivery for a 50-day period when anaerobic conditions were maintained. Two-year microcosm tests also confirmed long-term stability of the reduced uranium. Other field tests involving injections of dissolved oxygen (DO) or nitrate to the reduced-zone subsurface demonstrated reoxidization of U(IV) and remobilization of U(VI). Subsurface delivery of ethanol as an electron donor, however, effectively restored reducing conditions and decreased uranium concentrations to the previously low levels. Ongoing research involves additional characterization of U(VI) reduction by delivery of multiple or slowly-degrading electron donor sources and maintenance of long-term stability of immobilized uranium.
Monitoring results to date indicate that very low aqueous-phase concentrations of uranium can be achieved despite high solid-phase concentrations due to the low solubility of U(IV) and low rates of desorption/dissolution relative to the rate of reduction. Findings suggest that long-term bioremediation at S3 Ponds will need strategies for DO and nitrate control or methods to increase resistance of the immobilized uranium to remobilization by reoxidation.
Contributed by Craig Criddle, Ph.D (criddle@stanford.edu or 650-723-9032) and Wei-Min Wu, Ph.D (wei-min.wu@stanford.edu or 650-724-5310), Stanford University, and Philip Jardine, Ph.D (jardinepm@ornl.gov or 865-574-8058) and David Watson (watsondb@ornl.gov or 865-241-4749), ORNL.
Research Shows Growing Potential of Bioremediation for Arsenic and Selenium
Collaborative research among Duquesne University, the University of Pittsburgh, and the U.S. Geological Survey (USGS) is underway to identify bacteria and plant species with potential to improve bioremediation efficacy for arsenic (As) and selenium (Se). Bioremediation of these contaminants is an alternative to abiotic methods based on activated alumina, coagulation/filtration, lime softening, and reverse osmosis. Abiotic methods generally cost more, remove As(III) less effectively, require manipulation of pH, and produce more waste. Integrated laboratory and greenhouse test results show significant potential for arsenite-oxidizing, arsenate-reducing, or sulfidogenic bacteria as well as hyperaccumulating yeast (Saccharomyces) and brake fern (Pteris).
Many Pteris species such as P. vittata (ladder brake) and P. cretica (Cretan brake) have proven to hyperaccumulate As in the stems and leaves. Arsenic typically enters the plant as As(V) through phosphate channels in the roots, where it is reduced to As(III) and stored in other plant tissues. When accumulation of As(III) is complete, harvested plant tissues are disposed as hazardous waste.
In Duquesne/University of Pittsburgh greenhouse studies, P. cretica exposed to water containing 300 µg/L As(III) resulted in 100% oxidization to As(V) and subsequent plant uptake of As(V) within 100 hours (Figure 3). Rates of As uptake showed similar trends across Pteris spp. Arsenic uptake in control experiments with non As-accumulating Boston fern (Nephorlepsis exaltata) also showed rapid As(III) oxidation, but no uptake of As.
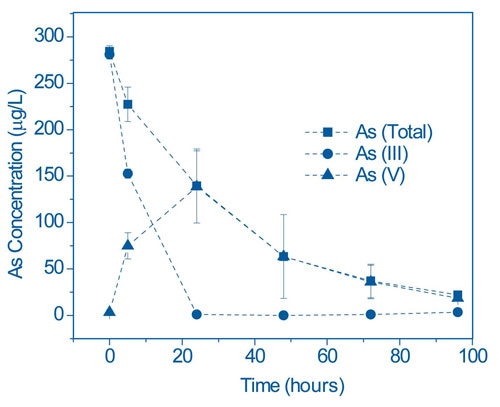
Researchers also are evaluating various changes in the rhizosphere of P. cretica. Addition of an antibiotic mixture to the water was found to significantly reduce microbial populations, reducing the As(III) oxidation rate by approximately 50% within 48 hours and consequently delaying As uptake. These results suggested that the rhizosphere is one of the controlling factors in As uptake. Testing now focuses on the use of specific nutrient amendments to enhance rhizosphere bacterial activity. microbiota is one of the controlling factors in As uptake. Testing now focuses on the use of specific nutrient amendments to enhance rhizosphere bacterial activity.
Selenium (Se) commonly accumulates as a result of erosion and industrial or agricultural runoff followed by evaporation in areas with extensive hydrocarbon content. It is frequently present in wastewater discharged by petroleum-processing facilities. Phytoremediation employing plant species that hyperaccumulate (selenium weed Neptunia amplexicaulis, and Astragalus spp. such as loco weed and milk vetch) or volatilize (Indian mustard Brassica juncea) has shown limited success with Se.
In contrast, more than 20 bacterial species have demonstrated capability to respire selenate, converting Se(VI) to Se(IV) and Se(0). Field tests show effective stimulation of selenate-respiring bacteria under anaerobic conditions, although co-contaminants such as nitrate commonly inhibit selenium reduction. Various methods for avoiding co-contaminant inhibition have been integrated in pilot-scale treatment systems. In the Panoche Drainage District of northern California, for example, subsurface drainage is dosed with algae and injected into settling ponds. This strategy successfully removes 80% of the Se through microbial precipitation but results in biomass accumulation.
The USGS/Duquesne study group evaluated a range of bacteria with potential for avoiding biomass accumulation during Se removal. Test results indicated that the soil bacterium Sulfurospirillum barnesii simultaneously reduces Se(VI) and respires nitrate. Washed suspensions of nitrate-grown cells removed more than 98% of the 50-µM Se(VI) as nanospheres of Se(0). The cells exhibited high affinity for both nitrate (0.7 µM) and selenate (21 µM) and two separate enzyme pathways.
Results from these studies will be applied in upcoming USGS field applications. Site-specific information about other projects addressing As and Se contamination are available at http://water.usgs.gov/nrp/proj.bib/oremland.html, http://toxics.usgs.gov/topics/rem_act/saco.html and http://pubs.usgs.gov/fs/fs-031-03/.
Contributed by John Stolz, Ph.D., Duquesne University (stolz@duq.edu or 412-396-6333), Radisav Vidic, Ph.D., University of Pittsburgh (vidic@engr.pitt.edu or 412-624-1307), and Ronald S. Oremland, USGS (650-329-4482)
EPA Releases Technical Resource for MNA of Inorganics
The U.S. EPA Office of Research and Development, in cooperation with the Office of Superfund Remediation and Technology Innovation and Office of Radiation and Indoor Air, recently published a two-volume technical resource for selection of MNA as a site-specific remedy component for inorganic contaminants in ground water. Volume 1, Technical Basis for Assessment, provides an overview of the technical basis for MNA of inorganic contaminants (EPA/600/R-07/139). Volume 2, Assessment for Non-Radionuclides, addresses technical aspects of attenuation mechanisms and data collection for arsenic, cadmium, chromium, copper, lead, nickel, nitrate, perchlorate, and selenium (EPA/600/R-07/140).
Together, the documents describe a tiered analysis applicable to MNA site screening through an iterative collection of site-specific data that progressively reduces MNA uncertainty. This analysis helps cleanup managers develop detailed information about site hydrogeology, mechanisms and rates of contaminant attenuation, aquifer capacity to sustain attenuation of contaminant mass, and long-term stability of immobilized contaminants. The documents also describe methods for determining attenuation mechanisms by measuring key ground-water chemical and physical parameters (including reduction/oxidation characteristics), identifying chemical speciation of contaminants and key reactants in ground water, and evaluating reactions between contaminants and solid components within the aquifer. Both volumes of Monitored Natural Attenuation of Inorganic Contaminants in Ground Water may be downloaded on CLU-IN at http://www.clu-in.org.





