Active Capping Demonstrated on Anacostia River
The Hazardous Substance Research Center/South and Southwest (HSRC/S&SW), a U.S. EPA-funded, university research consortium led by Louisiana State University, is demonstrating an active capping process to remediate portions of the Anacostia River in Washington, DC. The HSRC/S&SW is collaborating with EPA’s SITE program, members of the Anacostia Watershed Toxics Alliance, the EPA/Industry Sediment Remediation Technology Development Forum, and the District of Columbia government. Conventional sand caps are designed to reduce contaminant release from sediments by physically isolating contaminants from organisms and the water column. The active capping process underway at the Anacostia, however, involves covering contaminants with layers of alternative materials that offer treatment and/or sequestration of contaminants.
Large amounts of sediment wash, excess nutrients, industrial waste, and urban runoff have severely degraded water quality of the Anacostia. As a result, the river contains extremely low levels of dissolved oxygen and high levels of bacteria that detrimentally affect aquatic life and use of the river. Demonstration of active capping is occurring on a grid of capping cells located on several acres along the river west of the Washington Navy Yard.
Extensive site assessment was conducted in 2002 to identify the sediment contaminants and their distribution and to characterize the site’s hydrological and geotechnical properties. PCB concentrations in the demonstration sediment were found to reach 6-12 ppm, and total polycyclic aromatic hydrocarbons reached 30 ppm. In addition, elevated concentrations of target metals exist in the sediment: cadmium (3-6 ppm), chromium (120-155 ppm), copper (127-207 ppm), lead (351-409 ppm), mercury (1.2-1.4 ppm) and zinc (512-587 ppm). Although the flow velocity of the river is relatively low (less than 1 ft/s), the area is subject to 1-ft tidal variations and tidal influence seepage. Soft sediments with a surficial strength of approximately 10 lb/ft2 underlay much of the area.
Following two years of laboratory treatability studies, HSRC/S&SW selected four alternative cap technologies for the demonstration:
- AquaBlokTM: a permeability control agent tested in conjunction with the SITE program
- Apatite: a phosphate mineral with ability to scavenge metals
- Coke: a high carbon-content material capable of sorbing organic contaminants, and
- Laminated mat: synthetic materials emplaced under controlled conditions, with coke for added weight.
Other capping materials such as activated carbon, zero valent iron, and commercial sorbents were excluded due to cost, lack of effectiveness under the conditions of the Anacostia, or potential problems with placement or long-term performance.
The demonstration is taking place in five 100- by 100-ft study cells, each containing one of the four capping materials as well as a conventional sand control. Two- to six-inch layers of capping materials were placed and verified using underwater surveying techniques. Conventional clamshell buckets introduced the cap materials in thin lifts. This technique reduced loading on underlying sediment and minimized intrusion to the water column above, while safeguarding future use of the waterway and providing adequate contaminant isolation. Use of a global positioning system to guide the buckets ensured uniform placement of individual loads.
Field efforts began in March 2004 with placement of a sand cap, followed by the alternative cap materials. Preliminary results indicate that the simple technique of clamshell distribution can effectively lay the thin lifts of cap material required over soft sediment. Some variation in material thickness due to intermixing with soft sediment and to placement variations has been noted, but no areas of inadequate cap coverage have been detected. Final analysis of the cap placement effectiveness is expected in late spring.

Monitoring of the caps is underway to evaluate changes in chemical isolation, physical stability, and fate processes. Long-term monitoring of the site will continue to track sediment recovery and overall improvements to the Anacostia watershed. Preliminary performance of the capping materials will be evaluated in 2004 with monitoring continuing through at least 2005. Webcam viewing of the demonstration and project updates are available at http://www.hsrc-ssw.org/anacostia/.
Contributed by Danny Reible, Ph.D., Louisiana State University (225-578-6770 or reible@lsu.edu)
NRMRL Evaluates Active and Semi-Passive Technologies for Treating Acid Mine Drainage
The U.S. EPA National Risk Management Research Laboratory (NRMRL) cooperated with EPA/Region 9, the State of California, and Atlantic Richfield Corporation over the past three years in evaluating technologies for treating acid mine drainage (AMD) and acid rock drainage (ARD). Evaluations focused on three technologies used to treat drainage from mine workings and seeps at the Leviathan mine in Alpine County, CA: 1) active biphasic (two-step) lime treatment; 2) semi-passive settling in an alkaline (lime) treatment lagoon; and 3) passive compost-free bioreactors (Figure 2). Biphasic and lagoon treatment technologies enhance conventional alkaline-based technologies, which typically capture, store, and batch or continuously treat water through the addition of lime to neutralize water acidity and precipitate metals. In contrast, the compost-free bioreactor technology nurtures sulfate-reducing bacteria that generate sulfides capable of scavenging dissolved metals to form metal sulfide precipitates.

AMD is caused by sulfur and sulfide mineral oxidation occurring when oxygen and water contact waste rock and mineralized rock in mine workings. The acid generated through the mixing of water and sulfide minerals dissolves metals such as aluminum, arsenic, copper, iron, and nickel. The metals in solution create toxic conditions for fish and insects. Since the mid-1860s, intermittent extraction of copper sulfate, copper, and sulfur minerals from the abandoned Leviathan mine resulted in extensive AMD and ARD. When converting underground workings into an open pit mine during the 1950s, workers removed approximately 22 million tons of overburden and waste rock from the open pit mine and distributed them across the 253-acre site. Placement of overburden and waste rock in local creeks led to ARD, which when combined with AMD from the mine workings resulted in fish and insect kills in local creeks and the east fork of the Carson River.
State actions in 1984 significantly reduced the metals and acidity in AMD and ARD from the Leviathan mine. Actions included storm water controls, separation of Leviathan Creek from the waste rock, and construction of five ponds to prevent discharge of AMD. The Leviathan mine was added to the NPL in May 2000 to address AMD/ARD discharge to surface water. Cleanup has been hampered by the site’s alpine environment, which limits site access and operations each year from November through May.
The biphasic lime treatment used at the Leviathan mine employs two-step addition of lime to neutralize acidity and precipitate dissolved metals from the AMD at an inflow of 50-185 gpm. During the first step, the pH of AMD is raised from 2.8 to 3.2 by mixing it with lime slurry in a 10,000-gallon tank to precipitate iron as ferric hydroxide. Arsenic co-precipitates by adsorbing to the ferric hydroxide. The precipitate is flocculated and allowed to settle in a 10,000-gallon clarifier to form a small volume of arsenic-rich hazardous sludge. The sludge is dewatered through use of a filter press and shipped offsite for disposal. Water extracted from the sludge is recycled into the treatment system. In the second step, the pH of the partially-treated AMD is raised to approximately 8.0-8.4 in a second, 10,000-gallon tank, and the remaining metals are precipitated in an 800,000-gallon lagoon. The final precipitate forms a larger quantity of non-hazardous sludge that may be used onsite as soil amendment, pending the results of additional studies on sludge stability over time. The NRMRL study found that biphasic lime treatment results in metal removal efficiencies exceeding 99% (Figure 3).
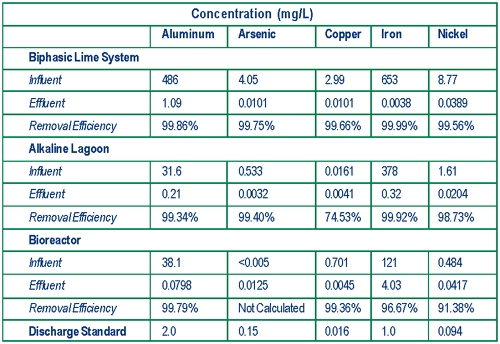
The alkaline treatment lagoon system at the Leviathan site is a simplified version of the biphasic treatment system employing a low-flow (12-30 gpm) ARD source containing low levels of arsenic. Single-step addition of lime combined with vigorous aeration in a series of three 1,000-gallon tanks neutralizes the ARD acidity (from pH 4.5 to pH 8) and precipitates dissolved metals. A series of 15- by 15-foot bag filters captures large flocc particles, and a 1.4-million gallon, multi-cell settling lagoon allows extended contact of ARD with the lime and additional time for fine particles to settle. The effluent is discharged to surface water. Tests showed that effluent discharge standards for all targeted metals typically are met following treatment in the lagoon system.
In late 2003, NRMRL began long-term evaluation of a compost-free bioreactor developed by researchers at the University of Nevada-Reno. The reactor relies on sulfate-reducing microbial organisms such as Desulfovibrio sp. to neutralize acidity and to precipitate metal sulfides from the ARD year-round at flow rates ranging from 8 to 30 gpm. Unlike compost bioreactors, this technology uses a liquid carbon source and a rock matrix (rather than a conventional compost or wood chip matrix) that is consumed by bacteria and collapses over time. Benefits of this technology include better control of biological activity and improved hydraulic conductivity and precipitate flushing.
The bioreactor treatment begins with the introduction of ARD to a pretreatment pond where sodium hydroxide is added to increase the pH from 3.1 to 4.0. Alcohols also are added to serve as a carbon source for the microbes. ARD flows from the pretreatment pond to an upstream, 12,500-ft3 bioreactor for biological reduction of sulfate to sulfide. Excess sulfide and partially treated ARD next pass to a second, 7,000-ft3 downstream bioreactor for additional metals removal. Both bioreactors contain 6- to 24-inch river rock aggregate that serves as a substrate for sulfate-reducing bacterial growth. Each bioreactor has three influent distribution lines and three effluent collection lines located at different elevations to allow variable flow operations.
Precipitates from the second bioreactor settle in a 16,400-ft3, continuous-flow pond. The effluent then flows to a rock-lined aeration channel that promotes degassing of residual hydrogen sulfide prior to discharge. Precipitate slurry is flushed periodically from the bioreactors to prevent plugging of the river rock matrix, and settled in an 18,000-ft3 flushing pond. Solids generated by this technology are non-hazardous and may be used (pending additional studies) as soil amendments during future reclamation of the site. Preliminary results indicate that this bioreactor system is achieving a metal removal efficiency of 91-99%.
NRMRL found that each technology promotes AMD/ARD neutralization and metal precipitation while meeting site discharge standards. The field studies suggest that active biphasic lime treatment may be more effective in applications involving a high rate of flow and a short treatment season, while the semi-passive alkaline treatment lagoon favors a lower flow rate and extended treatment season. The passive, compost-free bioreactor, however, is not constrained by seasonal conditions and can be scaled to treat the low to moderate flows common at AMD and ARD sites. In addition, both the biphasic and alkaline treatment lagoon technologies generated larger quantities of sludge than the bioreactor.
NRMRL will publish additional information such as implementation costs, benefits, and limitations of the two lime-based technologies in an ITER (innovative technology evaluation report) and demonstration bulletin later this summer. Documentation of the bioreactor evaluation will be available in mid 2005.
Contributed by Ed Bates, NRMRL (513-569-7774 or bates.edward@epa.gov) and Matt Udell, Tetra Tech EM Inc. (916-853-4516 or matt.udell@ttemi.com)
Pilot and Full-Scale ISCO Using Sodium Permanganate in Fractured Bedrock
Two-phase pilot testing of in-situ chemical oxidation (ISCO) using sodium permanganate (NaMnO4) was conducted in 2000-2001 at the Tenneco Automotive site in Hartwell, GA, to remediate a dissolved-phase trichloroethene (TCE) plume in a fractured bedrock aquifer. The ISCO pilot was initiated to identify an alternative to ground-water pumping and treatment in the off-facility plume, which was used for 12 years without significant success (but at significant cost). Difficulties in ground-water remediation at this site were compounded by off-facility migration of the plume through connective fractures to 52 adjacent residential and commercial properties. Pilot test results demonstrated that ISCO can effectively remediate chlorinated volatile organic compounds (CVOCs) in a fractured bedrock aquifer due to the geologic setting’s low demand by natural organic matter for oxidant material. The pilot test also highlighted the need for using an effective hydrogeologic conceptual model in this type of project. Pilot test results led to ISCO full-scale implementation during 2003.
Degreasing fluids containing CVOCs were released at the facility between 1956 and 1981. TCE was detected in ground water at concentrations reaching 240 µg/L in an onsite well and 330 µg/L in an off-facility commercial well. The pilot test site is situated in Piedmont metamorphic and granite rocks that have weathered into a 20- to 50-ft layer of residual soil (saprolite) near ground surface. Hydraulic conductivity of the saprolite is approximately 1x10-4 cm/s. A more permeable transition zone of partially weathered rock (PWR) exists beneath the saprolite and above unweathered bedrock, at a typical depth of 50-60 ft below ground surface.
Impacted ground water at the site exists primarily in the PWR, which has an average hydraulic conductivity of 1x10-2 cm/s (30 ft/day), with an average ground-water flow rate of 100 ft/yr in the off-facility plume area. A bifurcated TCE plume in the unconfined PWR aquifer extends approximately 1,800 feet west beneath the facility and nearly 1,700 feet north into adjacent, off-facility properties. Development of an effective conceptual model, which cost nearly $1 million, involved geologic and hydrogeologic characterization using remote sensing techniques, downhole geophysics, hydraulic testing, and ground-water modeling.
Early bench-scale testing on ground-water and soil samples collected from the site indicated that TCE degradation in the field would depend on both residence time and NaMnO4 concentration. Within six hours, a NaMnO4 concentration of 50 mg/L completely degraded 1,000 µg/L of TCE in ground water collected from an on-facility area of the plume (with higher overall concentrations). Laboratory tests also demonstrated that the oxidant demand from factors such as natural organic material, TCE concentration, and ionic composition would not limit TCE degradation in ground water. Pre-injection activities included bromide tracer tests to calculate the concentration of NaMnO4 injectant needed to achieve the target concentration of 50 mg/L within the primary injection well.
The Phase I pilot test was conducted on-facility using an existing pumping recovery well and a monitoring well located approximately 30 ft from the recovery well. The initial field injection employed one gallon of a 40% NaMnO4 solution that was injected into the subsurface through the monitoring well. ISCO monitoring included sampling for TCE and degradation products such as chloride and vinyl chloride in ground water extracted from the recovery well. Water quality parameters such as pH, temperature, dissolved oxygen, and oxidation reduction potential also were measured.
Phase I results indicated that the lowest TCE concentration corresponded to the highest measurement in oxidation reduction potential, indicating that TCE oxidation by the NaMnO4 had occurred. An increased chloride concentration also corresponded to the lowest TCE concentration, at an approximate ratio of 3:1. Contrary to bench-scale testing, complete TCE degradation in ground water did not occur due to continued contribution of TCE from radial flow of impacted ground water into the pumping well.
Phase II pilot testing was conducted using an existing off-facility monitoring well screened in the PWR and located on commercial property with the highest off-facility TCE concentration in ground water. Four additional PWR monitoring wells were installed upgradient of the existing well at 20-ft intervals. A total of 7,780 gallons with an average NaMnO4 concentration of 210 mg/L was injected into a single well (MW-112A) during four events in May 2001. Within two months, three additional injections occurred in the same well using significantly higher NaMnO4 concentrations averaging 5,600 mg/L. In addition, the individual injection volume was lowered from 1,750 to 500 gallons in order to eliminate potential displacement of impacted ground water. The final injection occurred one month later in an alternate well (MW-112C) using the same NaMnO4 solution and volume. A lower injectant intake rate was noted during the final injection, likely due to lower hydraulic conductivity of a less fractured area.
Phase II results indicate that TCE concentrations in each of the monitoring wells decreased significantly after the NaMnO4 injections (Figure 4). The most significant reductions in TCE concentrations occurred after the fourth injection. Marginal increases in TCE concentrations in downgradient wells after the first (large) injection likely were caused by impacted ground water flushing from upgradient sources, or by variation in TCE concentrations reflecting the aquifer heterogeneity and fracture distribution. Downward trends in TCE concentrations followed by sudden increases in two of the wells (MW-112 and MW-112D) indicate that TCE in the wells was displaced by the initially large injection rather than degraded.
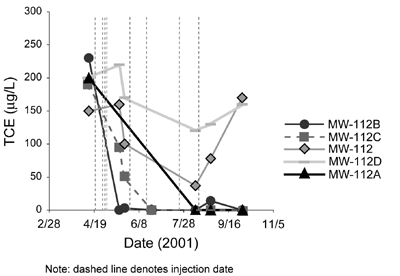
Overall pilot results indicated that no daughter products had formed, oxidant demand was minimal (less than 10 mg/L), and TCE concentrations were reduced to below the maximum contaminant level (5 µg/L) in three of the five wells. In addition, NaMnO4 was highly persistent in the aquifer (more than one year), no screen fouling was observed, and metal mobilization did not occur. The results of pilot-scale ISCO prompted refinement of the site conceptual model, including redefinition of the contaminant plume.
Current full-scale ISCO implementation builds upon lessons learned during the two pilot tests, the most important of which was the need to use relatively small injection volumes (only a fraction of estimated pore volume) in order to minimize displacement of treated ground water. Operations began with the installation of 12 new wells in the north off-facility portion of the plume. The first series of semi-annual injections was conducted in February 2003. Single injections ranging from 250 to 500 gallons in volume were accomplished through gravity feed of a 2% NaMnO4 solution to the screened zone of eight wells. Similar injection events were conducted in October 2003 and April 2004.
Preliminary results indicate that two monitoring wells in the vicinity of the injection well show TCE concentration reductions of 69 and 77%. TCE degradation in these wells is supported by the presence of oxidant degradation indicators such as increased levels of manganese dioxide and carbon dioxide and a temporary decrease in ground-water pH. Semi-annual injections of NaMnO4 are scheduled to continue through the end of 2007.
Contributed by Carrie Williams, GeoSyntec Consultants (cwilliams@geosyntec.com or 404-236-7306), and Elizabeth Penny, State of Georgia/Environmental Protection Division (elizabeth_penny@dnr.state.ga.us)
Bioaugmentation Tested on DNAPL Sources
The U.S. Navy began laboratory and field experiments at the Dover National Test Site in 2001 to evaluate the use of in-situ bioaugmentation in enhancing dissolution of tetrachloroethene (PCE) dense nonaqueous phase liquid (DNAPL). Bioaugmentation is an in-situ remediation approach in which selected microorganisms are injected in the presence of electron donors and nutrients to stimulate chloroethene dechlorination. Laboratory tests suggested that microbial cultures could be adapted to grow in high volatile organic compound (VOC) concentrations. In the field, naturally-occurring, dehalorespiring microbial consortia were introduced into DNAPL source areas within the saturated zone to function at the solubility limits of chlorinated solvents. Test results indicated that the microbes enhanced biodegradation rates at the DNAPL interface, thus increasing the concentration gradient driving DNAPL dissolution. Increasing the concentration gradient resulted in more rapid DNAPL dissolution and potential reductions in cleanup time and costs.
Both microcosm and column studies were conducted in a laboratory using soil and ground water from Dover Air Force Base. In microcosm tests, PCE degradation at solubility concentrations was evaluated using three different cultures. The two-dimensional (2-D) model aquifer column studies compared enhanced biostimulation and bioaugmentation. Column samples were analyzed for VOCs, dissolved gases, anions, volatile fatty acids, molecular assessment, and compound-specific isotopes. Screening of a range of electron donors over a period of 700 days resulted in the selection of ethanol as an electron donor.
No significant PCE biodegradation was observed throughout the biostimulation phase of laboratory testing. Results indicated that indigenous dehalorespiring bacteria (such as Dehalococcoides) in the aquifer material could not actively dechlorinate PCE. The mass discharge of total chlorinated ethenes in the bioaugmented model aquifer, however, was approximately 120% more than in the non-bioaugmented aquifer.
Field experiments were conducted at a Dover test cell where 100 L of PCE had been released. Ground water was recirculated in the cell at a constant velocity throughout three phases of testing: baseline, biostimulation, and bioaugmentation. Extensive monitoring networks of conventional and multilevel piezometers were employed to assess spatial and temporal trends in parent and daughter compound concentrations and mass transport. These data were complemented by microbial community analysis using molecular genetic methods and compound-specific isotopic analysis that confirmed dechlorination was occurring through anaerobic biodegradation.
As observed in the laboratory studies, biostimulation (using ethanol and lactate) in the field did not produce significant PCE reduction. Bioagumentation using 50 L of a bacterial culture (KB-1TM) resulted in an increase in trichloroethene, cis-1,2-dichloroethene, vinyl chloride, and ethene.
Standard and quantitative polymerase chain reaction (PCR and QPCR) analyses were used to assess bioaugmentation effects on the microbial community. While standard PCR estimated the intensity of microbial response compared to known standards, QPCR estimated the actual numbers of target microorganisms (as gene copies/L) in each sample. PCR analysis indicated that intensity scores in the test cell ranged from 81 to 261% of the control samples, which was significantly higher than the non-detectable levels (<3%) estimated prior to bioaugmentation. QPCR analysis indicated large increases in the numbers of Dehalococcoides approximately 100 days after bioaugmentation, with an average of 7.87 x 108 rRNA gene (16S) copies/L. These results support the increased concentrations of cis-1,2-dichloroethene, vinyl chloride, and ethene that were observed throughout the test cell.
Now that complete dechlorination to ethene is observed consistently in areas nearest the residual PCE zones, bioaugmentation enhancement factors will be calculated. Monitoring of the field test cell will continue under the interagency Environmental Security Technology Certification Program (ESTCP). Updated project information is available on-line at http://www.estcp.org/documents/techdocs.
Contributed by Carmen LeBron, Naval Facility Engineering Service Center (805-982-1616 or carmen.lebron@navy.mil) and Michaye McMaster, GeoSyntec Consultants (519-822-2230 or mmcmaster@geosyntec.com)





