- Monitoring Shows Enhanced Abiotic Degradation of TCE DNAPL Resulting from Emulsified ZVI Injection
- EPA Targets Key Areas of Nanotechnology Research
- High Specificity of Metal Detection Achieved by Nanocontact and Polymer Nanojunction Sensors
- Bimetallic ZVI Technology Implementation Expands to Remediate VOC Hot Spots in Ground Water
- Microscale Metal Sorbents Approach Commercial Use
- Federal Agencies Partner in Upcoming Nanotechnology Workshop
As a member of the interagency National Nanotechnology Initiative, the U.S. Environmental Protection Agency (EPA) is helping to coordinate research and development of nanoscale science, engineering, and technology. In this role, EPA also is studying the potential risks posed by manufactured nanomaterials due to their composition, reactivity, and unique size. This issue of Technology News and Trends focuses on emerging nanotechnologies applicable to hazardous waste characterization and remediation.
Monitoring Shows Enhanced Abiotic Degradation of TCE DNAPL Resulting from Emulsified ZVI Injection
Nanotechnology Resources
Within the framework of the National Science and Technology Council, the National Nanotechnology Initiative (NNI) oversees nanoscale research and development among 23 federal agencies. The NNI aims to maximize the potential of nanotechnology, facilitate nanotechnology transfer, develop education resources and an infrastructure for nanotechnology advancement, and oversee responsible development of nanotechnology. More information is available at http://www.nano.gov.
The National Aeronautics and Space Administration (NASA) has compiled nearly three years of monitoring data from the first field-scale demonstration to evaluate nanoscale emulsified zero-valent iron (EZVI) injections used for enhancing in-situ dehalogenation of dense, nonaqueous-phase liquid (DNAPL). Collaborating with researchers from the University of Central Florida and EPA, NASA initiated the project as part of a multi-prong effort to treat extensive areas of subsurface trichloroethene (TCE) contamination at Cape Canaveral Air Force Station, FL. The demonstration results show that this emerging technology has the potential to effectively treat DNAPL source zones and suggests capability for treating dissolved-phase contaminants in the vicinity of DNAPL.
EZVI technology capitalizes on the ability of food-grade surfactant, biodegradable vegetable oil, water, and ZVI to form hydrophobic emulsion droplets that are miscible with DNAPL (Figure 1). Chlorinated volatile organic compounds (VOCs) in DNAPL diffuse through the oil membrane and undergo reductive dechlorination in the presence of ZVI in the interior aqueous phase. Encapsulating ZVI in a hydrophobic membrane protects the nanoscale iron from other ground-water constituents such as inorganics that might otherwise use some of the iron's reducing capacity, and thereby reduces the mass of EZVI available to treat target contaminants and overall project costs. Additionally, EZVI's vegetable oil and surfactant components enable the material to serve as a long-term electron donor and promote anaerobic biodegradation.
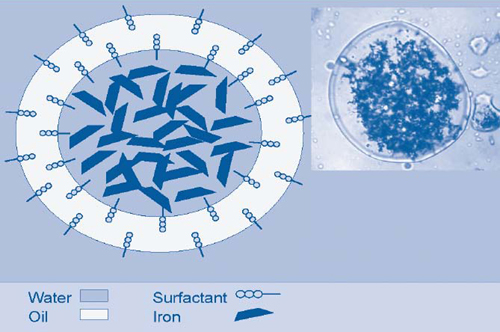
The demonstration was conducted in a 15- by 9.5-foot area below a building at Launch Complex 34. Pre-demonstration soil cores showed the presence of TCE in concentrations exceeding 250 mg/kg. EZVI was injected throughout a 10-foot-thick zone within the upper sand unit of the site's surficial aquifer system. The upper sand unit comprises medium- to coarse-grained sand and crushed shells and extends from the ground surface to approximately 18-25 feet below ground surface (bgs). The water table fluctuates from 3 to 7 feet bgs, with hydraulic gradients ranging from 0.09-3 ft/ft to 0.07-2 ft/ft.
Primary objectives of the demonstration test were to estimate changes in total TCE mass and TCE DNAPL mass in the target unit as well as changes in TCE flux to ground water. A total of 21 ground-water monitoring points were established within a system composed of a fully screened well and four monitoring wells with five sample intervals. Two additional monitoring wells were installed to obtain measurements at three depth intervals outside the treatment zone.
A ground-water control system with activated carbon was installed to create a closed-loop recirculation cell and forced gradient conditions across the treatment zone. The system operated for three weeks prior to the injection to allow for post-injection evaluation of flux caused by the treatment process. Along with baseline ground-water and soil quality parameters, pre-demonstration sample sets were measured for interfacial surface tension between EZVI and water, TCE and water, and EZVI, TCE, and water.
EZVI was introduced to the aquifer during a single injection occurring over five days in October 2002. Based on earlier laboratory evaluations, the selected injectant contained 44.3% water, 37.2% oil, 1.5% surfactant, and 17% (100 to 200 nm-sized particle) iron by weight. The mixture was injected into eight 3 inch-diameter wells at injection intervals of 16-20 and 20-24 feet bgs using pressure pulse technology. A total of 670 gallons of the EZVI mixture was injected. To enhance distribution of EZVI into the formation, additional ground water from a treatment zone monitoring well was added at the injection points.
Visual observation of four interim soil cores obtained two months after the injection, as well as laboratory analysis of six soil cores collected three months after injection, confirmed that EZVI reached some target areas but missed others and migrated upward from the treatment zone. The ground-water recirculation system operated for a three-week period five months after the injection in order to evaluate changes in contaminant concentrations and mass flux. To evaluate longer-term impacts of treatment, additional ground-water analytical samples were collected 19 and 22 months after the injection.
As anticipated, the demonstration results indicated that EZVI was miscible with the DNAPL and caused little downward movement of DNAPL. Analysis of soil samples showed an 80% average reduction of TCE concentration within 90 days at all but two boring locations, apparently due to upward migration of EZVI. The maximum individual reduction was noted in a soil boring taken at a depth of 16-18 feet, where the TCE concentration decreased from 6,067 to 1 mg/kg following treatment.
Geostatistical analysis indicated 58-85% reductions in total TCE and TCE DNAPL mass from initial pre-treatment estimates of 17.8 kg and 3.8 kg, respectively, in the upper sand unit. Analysis of TCE in ground-water samples collected from wells located in the center and downgradient portions of the treatment area indicated 57-100% reductions of TCE at all targeted depths and significant reductions in TCE degradation products. A maximum individual reduction was noted in ground water collected from a single well at a depth of 23.5 feet, where the TCE concentration decreased from 700 mg/L to less than 1.0 mg/L following treatment.
Analysis of TCE and biodegradation products suggested that TCE reductions in the demonstration area were caused by contaminant destruction rather than mobilization. Mass flux for a downgradient transect decreased approximately 56%, from 19.2 to 8.5 mmol/day/ft² over a period of 6 months. Slug tests within the treatment zone indicated only a minor change in hydraulic conductivity following treatment, from 43 ft/day to 38 ft/day, likely due to iron oxide buildup or biofouling.
The relatively wide range in post-treatment TCE concentrations suggested that EZVI was distributed unevenly during the initial fieldscale injection. To address this problem, a second field test was conducted in early 2004 to evaluate improved techniques for injectant delivery: pneumatic fracturing, hydraulic fracturing, pressure pulsing, and direct injection. Each of these technologies was used to deliver 100 gallons of EZVI containing nanoscale iron to depths of 16- 19-ft in an open field near the original injection location. Results showed that pneumatic injection and direct push technology allowed for more controlled injections without loss of EZVI above or below the target region. Overall findings of these tests recommend that full-scale applications include preliminary testing to confirm that planned injection methods do not damage emulsion droplets during the injection process.
Nearly three years of monitoring following the demonstration suggests that biodegradation enhanced by the presence of oil and surfactant in the EZVI emulsion has contributed to TCE reductions. Follow-on EZVI tests under the U.S. Department of Defense's Environmental Security Technology Certification Program (ESTCP) will evaluate the proportion of chlorinated solvent mass destruction that occurs due to abiotic degradation versus enhanced biodegradation stimulated by the addition of EZVI electron donors. The ESTCP project also will continue to evaluate unresolved issues associated with injection methods and DNAPL mobilization potential.
Contributed by Jacqueline Quinn, NASA (321-867-8410 or jacqueline.w.quinn@nasa.gov) and Thomas Krug, GeoSyntec Consultants, Inc. (519-822-2230 or tkrug@geosyntec.com)
EPA Targets Key Areas of Nanotechnology Research
Through its Office of Research and Development (ORD), the EPA.s National Center for Environmental Research (NCER) has funded 32 Science to Achieve Results (STAR) research grants for more than $11 million in the applications of nanotechnology to protect the environment. The program focuses on developing low-cost, rapid, and simplified methods of removing contaminants from surface water; new sensors that are more sensitive for measuring pollutants; green manufacturing of nanomaterials; and more efficient and selective catalysts. An additional 12 STAR research projects are underway to study potentially harmful effects of manufactured nanomaterials.
ORD also has awarded contracts to more than 20 small companies through its Small Business Innovation Research Program to develop and commercialize nanomaterials and clean technologies. Current research projects in ORD laboratories are examining topics such as the use of nanostructured photocatalysts as "green" alternatives to hydrocarbon oxygenation; nanomaterials as adsorbents, membranes, and catalysts for air emission control; and ultrafine particulate matter data for assessing impacts of nanomaterial manufacturing. More information is available from the NCER at http://es.epa.gov/ncer.
High Specificity of Metal Detection Achieved by Nanocontact Sensors and Polymer Nanojunction
Researchers at Arizona State University (ASU) are working under a STAR grant to develop high-performance, low-cost sensors for initial onsite screening of heavy metals in ground and surface water. To enhance field portability, the fully developed sensors will be miniaturized and wireless and employ a printed wire board capable of integrating data collected from several probes. Field testing of the prototype sensors is anticipated in 2007.
Each sensor consists of an array of nanoelectrode pairs on a silicon chip. Within each pair, the nanoelectrodes are separated by an atomic-scale gap created by quantum tunneling (Figure 2). Electrochemical deposition of only a few metal ions into the gap forms a bridge and provides nanocontact between the electrodes, thus triggering a quantum jump in electrical conductance. Different metals have different anodic deposition and stripping potentials. This allows the sensor to achieve high specificity by combining measurements such as redox potentials, point-contact spectroscopy, and electrochemical potential-modulated conductance changes.
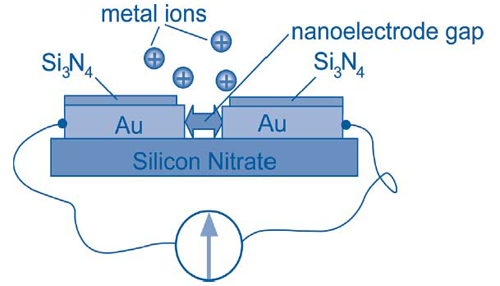
Results of preliminary tests on samples containing elevated copper concentrations showed that the deposition time needed to bridge the nanoelectrode gap decreased across samples as copper concentrations increased. Detection of copper at the EPA limit of 10-5 mol/L was achieved within 7 seconds, while 1-nanomolar concentrations were detected in 16 seconds. Similar results were observed in lead detection tests.
This approach can be enhanced through use of peptide-modified polymers that change conductance between the nanoelectrodes in the presence of heavy metal ions. When integrated, these polymer nanojunction sensors obtain more sensitive and real-time measurement of heavy metals in ground or surface water with higher accuracy.
Performance testing of laboratory-based models of the nanocontact sensors involved measurement of copper in drinking water samples collected from different surface water sources. Sensor data were compared to information measured by conventional laboratory techniques such as atomic absorption spectrometry with a 90% correlation between the data sets. Integrated results from the nanocontact and polymer nanojunction sensors were obtained in approximately 96 and 97% less time, respectively, than those gathered from multiple laboratory procedures.
ASU researchers are developing a similar field-portable sensor for use in monitoring air quality and arsenic in ground water, as well as other molecular- and nanojunction-based chemical and biological sensors based on carbon nanotube and polymer technologies. Under a second STAR grant, the group is investigating the fate, transport, transformation and toxicity of manufactured nanomaterials of drinking water.
Contributed by Erica Forzani, Ph.D., Nongjian Tao, Ph.D., and Paul Westerhoff, Ph.D., ASU (480-965-9058 or erica.forzani@asu.edu)
Bimetallic ZVI Technology Implementation Expands to Remediate VOC Hot Spots in Ground Water
The Naval Air Engineering Station (NAES) in Lakehurst, NJ, is conducting a series of bimetallic nanoparticle (BNP) applications to treat ground water containing chlorinated solvents. Initial efforts to remediate the site involved a pilot system for injection of oxygen and propane to promote microbial degradation of the contaminants. Unsuccessful pilot results led to a 2001 treatability study and 2002 pilot test to evaluate BNP technology as an alternative remedy. Based on successful results, a full-scale application was completed in 2003 to treat two contaminant source areas at the facility. Six rounds of post-injection ground-water sampling demonstrated the technology's effectiveness, and NAES now is planning additional BNP work to be implemented next year. The follow-on work will expand the area of treatment while applying lessons learned during earlier applications.
Full-scale BNP injections were conducted in an area with two distinct contaminant hot spots of a plume spanning approximately two miles. The area contains unconsolidated sediment extending approximately 75 feet bgs and consisting of 86-94% sand mixed with gravel and clay. Total organic carbon levels range from 40 to 800 mg/kg. Hydrologic evaluation of the area estimates a ground-water velocity of 0.59 ft/day with a hydraulic gradient of 0.002 ft/ft.
The plumes consist primarily of tetrachloroethene (PCE), TCE, and 1,1,1- trichloroethane (TCA) and degradation products such as cis-dichloroethene (cis-DCE) and vinyl chloride. Contamination extends 70 feet below the ground water table, which is located approximately 10 feet bgs, with greatest concentrations at a depth of 45-60 feet below the water table. The highest total VOC concentration in ground water prior to treatment was approximately 900 µg/L.
The selected BNP slurry contained iron particles averaging 50 nm in diameter and coated with trace (0.1% by weight) palladium that served as a catalyst for redox reactions. The slurry was injected directly into the aquifer across the treatment area over a period of 13 days using direct-push technology at 15 injection points. The injections were conducted in a grid pattern for one portion of the plume and in a linear pattern for the second portion in order to form a treatment wall along the facility boundary. At each injection point, twenty pounds of BNP were mixed with 1,200 gallons of water and injected at a rate yielding a BNP concentration of approximately 2 g/L. A Geoprobe® distributed the mixture over a 20-foot depth interval to reach a total treatment depth of 70 feet bgs.
The first round of post-treatment sampling of ground-water monitoring wells took place the following week, and the final round occurred nearly five months later. Eleven monitoring wells were used to monitor BNP effectiveness in the grid-injected portion of the treatment area. Additional wells located upgradient and downgradient of the treatment area were used to monitor effectiveness of the linear injections and local conditions.
Contaminant concentrations decreased throughout the monitoring period with the exception of brief VOC elevations in approximately 50% of the monitoring wells one week after the injections were completed. The temporary increases likely were caused by BNP-induced VOC desorption from soil particles to the aqueous phase. Overall, TCE and DCE concentrations decreased an average of 79% and 83%, respectively, and the average total VOC concentration decreased 74% (Figure 3).
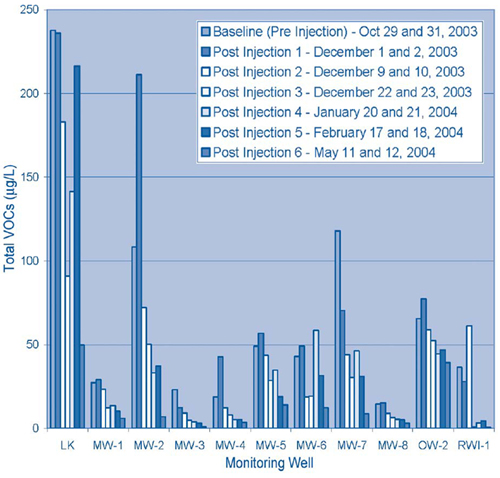
All samples were analyzed in the laboratory to determine VOC, chloride, iron, and dissolved iron concentrations as well as ground-water parameters such as oxidation/reduction potential (ORP) and pH. During the pilot test, ORP levels reduced from a range of +170 to +311 mV prior to treatment to a range of -100 to -400 mV after the injection process. Significant reducing conditions were observed for up to three months following the injections.
Post-injection testing indicated a BNP distribution of approximately 20-30 feet from each injection point. BNP reactivity was exhausted approximately nine months after the injections, at which point an approximate 50% rebound in contaminant concentrations was observed. The next round of injections will employ a higher concentration of BNP in order to expand the area of treatment and more completely reduce contamination. Remaining plume contamination will be addressed through natural attenuation.
For the BNP work already completed, NAES estimates a cost of $20,000 for bench-scale testing, $60,000 for pilot testing, and $250,000 for full-scale testing. Costs for full-scale implementation will vary significantly, however, based on the size and conditions of the treatment area and the concentrations of VOCs to be treated.
Contributed by Mike Figura, NAES Lakehurst (732-323-4857 or michael.figura@navy.mil) and Harch Gill, PARS Environmental, Inc. (609-890-7277 or hgill@parsenviro.com)
Microscale Metal Sorbents Approach Commercial Use
The U.S. Department of Energy (DOE) Pacific Northwest National Laboratory (PNNL) is working with private industry to expand field application of sorbents for removal and stabilization of specified metals from contaminated liquid and nonaqueous media. Known as "SAMMS" (self-assembled monolayers on mesoporous supports), the technology employs mesoporous (20-200 Å) materials with a large enough pore size to allow attachment of a monolayer and increased access to pore binding sites. The material's high surface area (approximately 1000 m²/g) also allows high densities of binding sites. Five grams of SAMMS powder, for instance, can provide a surface area equivalent to a football field on which binding molecules react. Bench- and field-scale tests on mercury samples indicate that the technology provides high metal loadings, high metal affinities, and rapid kinetics.
Both the monolayer and the mesoporous support can be tailored for a specific application. For example, the functional group at the free end of the monolayer can be designed to selectively bind targeted ions or molecules while the pore size, monolayer length, and density can be adjusted to give the material specific physical properties such as wettability and diffusion. Based on the completed laboratory and field testing of "Thiol- SAMMS," which was designed specifically for mercury removal, PNNL researchers have compiled aqueous performance data on key criteria such as maximum metal loadings (Figure 4).
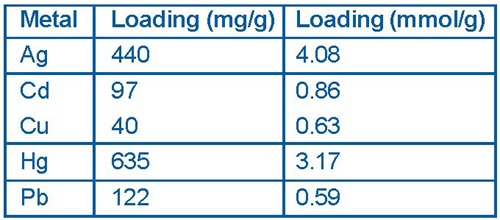
The initial large-scale field demonstration of SAMMS was conducted during 2000 at DOE's Mound Site to treat mercurycontaminated oil in storage drums. Analysis of the stabilized sludge solids and oil following 24-hour contact with SAMMS indicated that treatment successfully reduced concentrations of mercury in the oil from 4 mg/kg to below the RCRA leachability limit of 0.2 mg/L.
Application lessons learned from the Mound demonstration were employed the following year to test the technology in stabilization of more concentrated and co-contaminated mercury in containerized vacuum pump oil from Sandia National Laboratory. Testing at Oak Ridge National Laboratory showed that exposure to stabilized SAMMS for two months allowed for successful absorption of mercury in concentrations of 540 mg/kg and stabilization of waste oil meeting RCRA land disposal restrictions.
More recently, PNNL conducted benchscale treatability tests of Thiol-SAMMS using metals-contaminated aqueous waste from a pilot-scale waste glass melter operation. Waste selected for this test included soluble mercury concentrations of 4.6 mg/L and high concentrations of iodide ion, which strongly complexes mercury. Test results indicated that SAMMS contact with pre-filtered waste solution removed approximately 99% of the mercury and achieved the RCRA mercury waste discharge limit of 0.15 mg/L.
PNNL anticipates that SAMMS soon will be available in other engineered forms such as beads, membranes, and membrane cartridges. More information is available from PNNL at http://samms.pnl.gov.
Contributed by Glen Fryxell, PNNL (glen.fryxell@pnl.gov)
Federal Agencies Partner in Upcoming Nanotechnology Workshop
On October 20-21, 2005, nine federal agencies will convene a Nanotechnology for Hazardous Waste Site Remediation Technical Workshop at the U.S. Department of Commerce in Washington, DC. The open workshop will serve as a:
- communication and scientific reporting forum on remediation research and technologies;
- stimulus for increased collaborations among researchers and government scientists; and
- forum for discussing research needs, barriers, and incentives for using new technologies to reduce environmental pollutants.
To register or obtain more information about the agenda, visit http://www.scgcorp.com/nanositeremed/index.asp.





