|
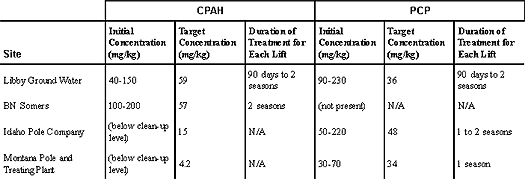
June 2001 Land Treatment Used at Wood-Treating Sitesby James C. Harris, P.E., U.S. EPA/Region 8 Engineered land treatment units (LTUs) have been employed to biodegrade contaminants resulting from past releases of wood-treating fluids at four former wood-treating Superfund sites in Montana. Since the mid 1980s, the U.S. EPA’s Region 8 office has been working with property owners to design, construct, and operate the LTUs, which are located at the Libby Ground Water site in Libby, the BN Somers site in Somers, the Idaho Pole Company site near Bozeman, and the Montana Pole and Treating Plant site near Butte. Since construction of the first LTU in 1989 and the fourth in 1996, extensive operation and performance data have been studied. Results show that remediation goals for soil containing pentachlorophenol (PCP) and carcinogenic polynuclear aromatic hydrocarbon (CPAH) compounds, which are the primary contaminants of concern, have been or are nearly achieved at these sites. The LTUs examined in this study treat contaminated soil volumes ranging from 19,000 cubic yards at the Idaho Pole Company site to 208,000 cubic yards at the Montana Pole and Treating Plant. At each site, contaminated soil from source areas was excavated and placed in an LTU where the soil was irrigated, tilled, and fertilized during growing seasons. Each LTU, which is rectangular or pie-shaped to facilitate irrigation, varies in size from 1 to 14 acres and accommodates a contaminated soil layer ranging from 0.5 to 7 feet in depth. To optimize soil moisture and control dust, a center-pivot irrigation system was installed at three of the sites, while berm-mounted, hydraulically-controlled sprinklers were used at the fourth. The optimum moisture content for the LTUs was found to be 70-80 percent of field capacity (12-15 percent water content). Early study information gained at the Libby Ground Water site indicated that moisture content may be the most important limiting factor in bioremediation of soil contaminated with PAHs. To increase the rate of oxygen uptake of naturally occurring microorganisms in the soil and to provide continual mixing of the treatment zone, the LTUs were tilled at the beginning of annual growing seasons. (Each growing season in Montana lasts approximately 90-120 days between June and September.) Earlier studies had indicated that the amount of soil capable of being treated to a remediation level in a specified time period is dependent on the size of the LTU and the depth of soil treated with each lift of a tiller. To maximize the depth of each lift at these sites, an 8-wheel drive, 200-horsepower tractor was used to pull a subsoiler plow with 3-foot tines. As a result, potential treatment depths were extended to 2.5 feet. Nitrogen and phosphorous in the form of agricultural fertilizer were added as nutrients to each LTU to stimulate microorganism performance. Calculations of the amounts of nitrogen and phosphorous to be applied at each LTU were based on soil carbon contents measured through analytical sampling. At the Montana Pole and Treating Plant site, liquid nutrients can be applied easily through the irrigation system, but other sites require granular fertilizer application through a spreader and tilling system. Since remediation levels for each soil lift are normally reached before the nutrients are depleted, nutrients typically are added only once to a lift of soil. The time required to achieve remediation levels has been found to vary with the type of wood-treating fluid used by the operating facility, as well as site-specific factors such as soil type, weather, and operational characteristics of the LTU system. Table 1 provides LTU performance information for each study site. Table 1. LTU Performance
Summary It is anticipated that complete remediation of the 45,000 cubic yards of CPAH- and PCP-contaminated soil at the Libby Ground Water site will be achieved by 2003, following 14 years of system operation. This estimate reflects installation of a second LTU in 1998 that reduced remediation time by 16-18 years. At the BN Somers site, where 67,000 cubic yards of CPAH-contaminated soil was treated through three lifts, remediation levels were achieved in 2000 after six years of LTU operation. Soil remediation levels for PCP were reached at the Idaho Pole Company in 2000 following five years of treatment. This time reflects an additional 3,000 cubic yards of contaminated soil that were treated through a single lift as follow-up to closure of the site’s wood-treating facility in 1999. At the Montana Pole and Treating site, approximately 52,000 cubic yards of contaminated soil have been treated since 1997, with the first 30,000 cubic yards reaching PCP-target concentrations in 1998 after a single lift treatment. Based on information gained from this study, it is estimated that the average cost of soil treatment in an LTU varies from $16 to $25 per cubic yard. For more information, contact Jim Harris (EPA/Region 8) at 406-441-1150 or harris.jim@epa.gov. Biological Treatment of Amine Wastes from the Gas Industryby J.R. Gallagher and J.A. Sorensen, University of North Dakota/Energy & Environmental Research Center The fate and remediation options for treating subsurface contamination from the natural gas industry have not been examined to great extent in the past. To address these concerns, a research project funded by the Canadian Association of Petroleum Producers, Canadian Occidental Petroleum Ltd, the U.S. Department of Energy, Gas Research Institute, Environment Canada, and the National Energy Board of Canada was initiated in 1995. Researchers at the University of North Dakota’s Energy & Environmental Research Center began studying related site-specific issues and remediation options for soil contaminated with amines, amine byproducts, and salts at a decommissioned gas plant near Calgary, Alberta. Preliminary studies on the biodegradability of amines, coupled with the relatively low mobility of amines, indicated that bioremediation was a remedial option at this site. Based on these findings, a 500 cubic-meter biopile including aeration, irrigation, and leachate collection systems was designed and constructed. After five months of active biotreatment and eight months of leaching, treatment had removed all identified amines and significant amounts of organic and inorganic nitrogen species, salts, and organic carbon. Environmental assessments conducted in 1992 as part of the gas processing plant decommissioning process revealed a layer of sludge-contaminated soil at a depth of 2-3 meters in the area of the plant’s former amine sludge disposal pits. During evaluation of potential biodegradation technologies, the site’s climatologic and geologic characteristics served as significant factors. The climate in this region is semi-arid to moist, with an average annual precipitation of 488 millimeters (mm) (19 inches) per year. Average temperatures typically are below 10ºC during 8-9 months of the year, with a frost-free period of 75-90 days. Other remediation options that were considered but found to be much more costly included landfilling of the contaminated soil or incineration followed by soil washing. Construction of the biopile (Figure 1) was completed over an 8-day period in July 1998. The completed containment cell measured 40 meters long by 10 meters wide and 1.5 meters deep. Above a 25-mil reinforced polyethylene bottom liner in the cell was a thin layer of crushed gravel covered by a filter fabric. The overlaying soil layer was mounded and enclosed by another liner. Approximately 450 cubic meters of treatment soils were housed within the constructed cell.
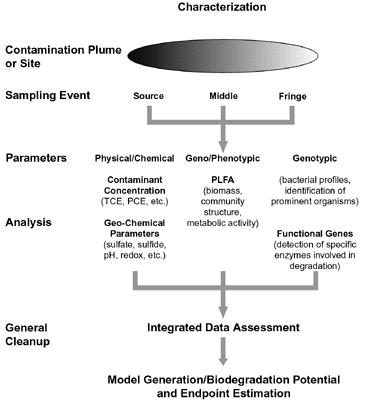
Soil additives to the system included 2.58 cubic meters of calcium chloride to increase soil permeability, as well as 2,036 kilograms of 10-34-00 (percent nitrogen-phosphorus-potassium) liquid fertilizer for increasing the microbial population and consequent biodegradation rate. To act as a bulking agent for increased porosity and permeability in the biopile, 50 cubic meters of straw were added within the treatment cell. One 100-mm, perforated polyvinyl chloride air vent powered by an external blower unit provided system aeration, along with four equally spaced, 50-mm flow ducts running the entire length of the cell. Microbial activity was enhanced by the addition of water through an irrigation system comprising five semipermeable hoses extending the cell length and powered by an external fresh water supply. The frequency and amount of water application were determined by weekly soil moisture measurements. Excessive salt leachate was collected in a sump located directly below the crushed gravel layer, temporarily stored in a reinforced external tank, and ultimately disposed in an onsite injection well. Following approximately three months of treatment in 1998 and two months in 1999, data indicated that biodegradation of the amine-related materials likely was complete. The remaining material was considered to be leachable but not biodegradable. At that point, the system began operating in a leaching mode and continued in this way for the remaining two months of treatment in 1999 and four months in 2000. Over the course of leaching mode operation, approximately 85,000 Imperial gallons of water (approximately 3 pore volumes) were applied to the biopile. Soil sampling was conducted at project start-up and bimonthly throughout the active treatment periods. Key soil character parameters used to evaluate general activity of the biopile included total Kjeldahl nitrogen (TKN, a measure of both ammonia and organic nitrogen), ammonia nitrogen (NH3-N), nitrate plus nitrite nitrogen (NOx-N) compounds, and total organic carbon. Based on the results of 20 sampling events over the course of treatment, data showed that TKN and total organic nitrogen concentrations decreased, while concentrations of ammonia and NOx-N (the byproducts of alkanolamines and other organic nitrogenous compounds) increased. During final stages of the study, however, TKN and total organic carbon levels remained steady while ammonia and nitrogen compound levels dropped significantly, thus indicating that the biodegradation of alkanolamines and the formation of thermal/oxidative products were complete. Final analysis showed that alkanolamine concentrations were reduced to levels below the detection limit following treatment, from an initial concentration of 15,000 mg/kg. The estimated cost of treating contaminated soil at this site through use of the biopile was $45 per cubic meter ($34.40 per cubic yard), exclusive of engineering and analytical costs. Researchers estimate that this cost could be reduced further in large-scale applications and if containment liners are not required. For more information, contact J.R. Gallagher (University of North Dakota) at 701-777-5030 or jgallagher@undeerc.org. Integrated Analytical Approach for Determining Bioremediation Effectivenessby D. Ringelberg, U.S. Army Engineering Research and Development Center, and A. Peacock, University of Tennessee/Center for Biomarker Analysis Researchers at the U.S. Army’s Engineering Research and Development Center and the University of Tennessee’s Center for Biomarker Analysis have developed a holistic approach to evaluating the potential for bioremediation at sites with contaminated soil. Dredged harbor sediment contaminated with polycyclic aromatic hydrocarbons (PAHs) resulting from past fuel releases was removed from the Milwaukee Confined Disposal Facility near the South Milwaukee Harbor in Wisconsin and examined for in situ biodegradative capacity. By integrating analytic chemistry, microbiology, and molecular biology techniques, the successional characteristics of indigenous microbiota were determined during a four-month bioslurry evaluation. Bench-scale testing showed that an intrinsic biodegradation potential of 52 percent for total PAHs was possible. In addition, soil chemistry at the facility indicated that PAH degradation likely would not be enhanced by other biological means. This integrated approach focuses on the use of “whole-sample” characterization techniques or direct assays of microbes in their natural environments, without impacting them during the measurement process. The approach eliminates the bias introduced when using a selected medium for the enrichment of native organisms, and overcomes the inability to culture a significant percentage of the native microbiota (90-99 percent of which are not culturable using standard bacteriological techniques). In addition, these techniques collectively provide the activity measurements or phenotypic/genotypic descriptions necessary for defining in situ biodegradation potentials, which no single assay can address. In this comprehensive process (Figure 2), unique soil chemical markers such as phospholipids and nucleic acids are recovered quantitatively at the contaminated site and subjected to analysis. Phospholipids, which constitute the mass bulk of most microbes, are used as an index of biomass, while microbial membrane lipids analysis is an effective tool for monitoring microbial responses to their environment. Analysis of phospholipids fatty acids (PLFA) profiles, which reflect both the nature of the intracellular components and the extracellular environmental conditions, indicates what types of microbes (i.e., bacteria, fungi, and algae) are present in a system, and how the microbes are reacting to environmental factors such as pollution and physical disturbances.
Nucleic acids also are used to provide the phylogenic specificity necessary for precise species identifications. Once microbial DNA is recovered, unique subsets of organisms can be targeted to define the diversity associated with that particular group of microbes. The diversity pattern that emerges from one soil sample can be compared to that of another to determine the co-occurrence, novelty, or absence of particular microbes across a site. During the South Milwaukee Harbor bioslurry evaluation, PLFA profiles, multiplex polymerase chain reaction (PCR) of targeted genes, and radio-respirometry techniques were used to define in situ microbial phenotypic, genotypic, and metabolic responses, respectively. Microbial PLFA analysis revealed a three-fold increase in biomass during the slurry test, and the resulting microbial community composition showed a strong correlation with observed changes in PAH chemistry. Genes encoding PAH-degrading enzymes increased by as much as four orders of magnitude, and changes in gene copy numbers showed strong correlations with shifts in specific subsets of the extant microbial community. Specifically, declines in the concentrations of the three-ring PAH components correlated with PLFA indicative of certain gram-negative bacteria (i.e., Rhodoccoccus sp. and/or actinomycetes) and genes encoding for naphthalene, biphenyl, and catechol degradative enzymes. Results of this study suggest that the intrinsic biodegradative potential of a contaminated site can be derived from polyphasic characterization of the in situ microbial community. This approach gives project managers a greater understanding of the processes involved in the biodegradation of targeted analytes, and the capability to build remediation models and conduct treatment tests in native soil without manipulation. Additionally, these techniques can result in significant cost savings when compared to traditional methods requiring analytical results from a larger number of samples taken across a full site. For further information, contact Dave Ringelberg (U.S. Army Corps of Engineers/CRREL) at 603-643-4744 or David.B.Ringelberg@erdc.usace.army.mil or Aaron Peacock (University of Tennessee/Center for Biomarker Analysis) at 865-974-8014 or apeacock@utk.edu.
|
|
|